flyp pe1200m User manual

USER: READ THIS GUIDE BEFORE OPERATING THIS DEVICE.
SAVE THIS GUIDE FOR FUTURE REFERENCE.
Portable Vibrating
Mesh Nebulizer
User Guide
Model pe1200m
23
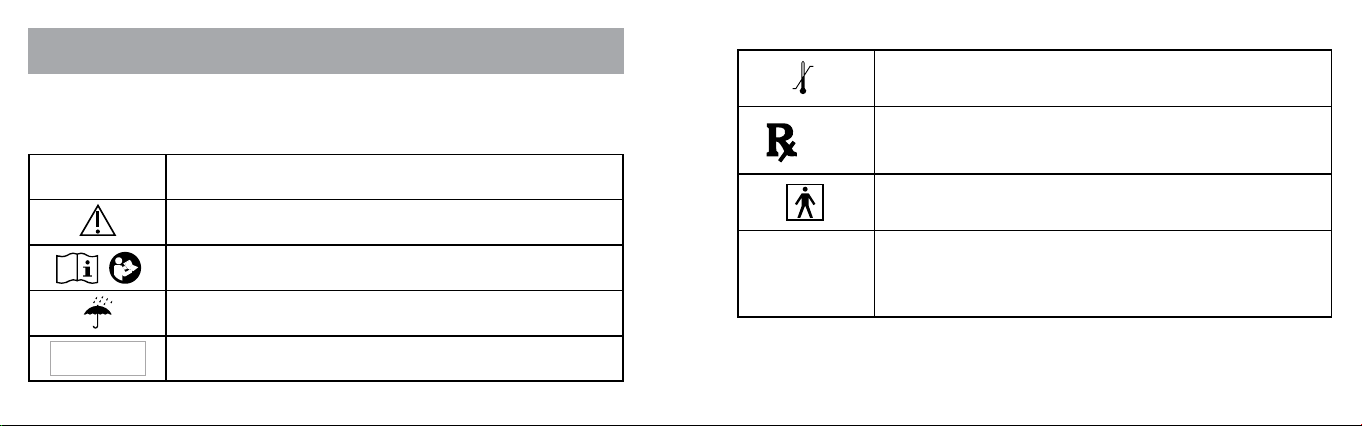
The symbols that appear on the box and the FLYP™ nebulizer are
described below:
SYMBOL MEANING
Caution
Consult instructions for use
Keep dry
Do not place in dishwasher
Transient storage temperature limits -10˚C to 45˚C
(14˚F to 113˚F)
Federal law (USA) restricts this device to sale
by or on the order of a physician or licensed
practitioner.
Type BF applied part
IP22
Protected against insertion of ngers and will not
become damaged or unsafe during a test in
which it is exposed to vertically dripping water
when held at an angle.
-10ºC
+45ºC
only
DO NOT PLACE
IN DISHWASHER
-10ºC
+45ºC
only
DO NOT PLACE
IN DISHWASHER
-10ºC
+45ºC
only
DO NOT PLACE
IN DISHWASHER
-10ºC
+45ºC
only
DO NOT PLACE
IN DISHWASHER
SYMBOLS
-10ºC
+45ºC
only
DO NOT PLACE
IN DISHWASHER

45
Important information
to prevent damage to your
FLYP™ nebulizer
Important safety information
regarding hazards that may cause
personal injury
Before using your FLYP nebulizer for the rst time, please read this
User Guide and clean the parts. Be aware of all warnings and safety
information. Only use accessories approved by Convexity and referenced
within this manual. If you do not fully understand all the warnings, safety
precautions, and operating instructions, contact Convexity for technical
support. ALWAYS KEEP THIS USER GUIDE HANDY.
This page left blank intentionally.
TABLE OF CONTENTS
Section 1: Introduction
Intended Use 8-9
Section 2: Safety Guidelines
Safety Guidelines 10-15
Section 3: Product Description
Features and Benets 16-17
Section 4: FLYP™ at a Glance
Names and Functions of Parts 18-20
Section 5: Using FLYP
About FLYP’s Battery 21-23
Filling Medication Reservoir with Prescription 24-26
Turning FLYP ON and OFF 26-27
Inhaling Prescribed Medication 28
Section 6: Cleaning and Disinfecting
Cleaning Parts 29-32
Disinfecting Parts 33-35
Section 7: Troubleshooting
Troubleshooting 36-37
Section 8: Support
Learning More 38
Section 9: Information
Technical Data 39
Specications 39-40
Aerosol Performance 41-42
Electromagnetic Compatibility 43-52
Disposal and Recycling 53
Warranty 53
Section 10: Replacement Parts and Accessories 54

89
INTENDED USE
Indications for use:
The FLYP™ nebulizer, for use by
adolescent and adult patients, is
intended to aerosolize healthcare
provider-prescribed solutions for
inhalation that are approved
for use with a general-purpose
nebulizer. FLYP is intended for
use at home or a medical facility,
such as a hospital or doctor’s ofce.
Intended user:
FLYP nebulizer, for use by
adolescent and adult patients,
should only be used by a patient
under the supervision of a qualied
medical expert, such as a doctor,
nurse, or respiratory therapist.
The user, or their actively assisting
caregiver, should be capable
of understanding all of the User
Guide’s contents. FLYP is intended
for use by a single user. FLYP is not
a life-saving device. Patients who
are in severe respiratory distress,
who are unconscious, or who
are not breathing spontaneously
should not use this device.
In an emergency, call 911
immediately for medical
assistance.
Caution:
Federal law (USA) restricts this
device to sale by or on the
order of a physician or licensed
practitioner.
Precautions:
All warnings and cautions
described in the User Guide should
be observed.
Service life:
FLYP has an expected service life
of 36 months.
Section 1: INTRODUCTION
10 11
SAFETY GUIDELINES
Read this section to learn how to
use FLYP™ safely and correctly
and to prevent risks and injuries
to you and others.
Keep this User Guide handy for
future reference.
Failure to follow these instructions
could result in serious injury or
damage to the device or other
property. Read all the safety
information below before using
the device.
Medical device:
FLYP is a medical device,
available only by prescription
and for prescribed medications.
Be sure to follow a qualied
medical expert’s instructions.
Direct exhaled medication away
from others.
If you are using FLYP to treat a
serious condition, a back-up
device is recommended.
However, in a life-threatening
situation, NEVER rely on a
nebulizer. Call 911 immediately
for emergency medical
assistance.
Use by others:
For single patient use only.
If others use it, infection may
spread.
Medical use:
FLYP is intended solely to deliver
prescribed medication to treat
a respiratory condition. Using
FLYP for any other purpose is
dangerous, and neither the
distributor, manufacturer, nor
their afliates can be held liable
for any damage or injury caused
by improper use or misuse.
Supervision required:
Adult supervision is required when
the device is used by children and
the inrm. If someone swallows
small pieces, such as the Stopper,
consult a doctor immediately.
You should also be aware that
the USB Wall Charger presents a
potential choking hazard.
Cleaning:
Be sure to clean the Mouthpiece,
Medication Reservoir and Stopper
before using them for the rst
Section 2: SAFETY GUIDELINES
12 13
time and after each subsequent
use (refer to pages 29-32).
The Main Unit should be cleaned
every day, but it should
NEVER
be
placed in water or washed in a
dishwashing machine.
ALWAYS
disconnect and unplug the USB
Wall Charger before cleaning
the Main Unit.
Do not drop medication on the
Main Unit or into its USB port. If
you drop medication on either
area, immediately wipe it off
with gauze. If you use FLYP™
while it is still wet, it may cause
trouble or injury.
Battery:
The battery is not replaceable.
Don’t attempt to replace the
battery yourself, as you may
damage it. Overheating and
injury could result. The lithium-
ion battery should be replaced
only by Convexity, and it must
be recycled or disposed of
separately from household
waste. NEVER incinerate the
battery. Incineration may cause
the battery to rupture. If an
ignition source exists, then re
and even an explosion could
result. NEVER immerse the
battery in water, as this may
cause the battery to rupture.
Electronic device:
FLYP complies with all applicable
electromagnetic compatibility
(EMC) standards. You should,
however, avoid operating it near
other electronic devices.
If you are not going to use the
unit for a long period of time,
disconnect the USB Wall Charger.
Charging:
FLYP contains an internal, lithium-
ion rechargeable battery that
cannot be removed.
Charge FLYP only with the USB
Wall Charger provided. NEVER use
the device when it is charging.
Battery life should exceed 500
charge cycles. All batteries
deteriorate over time if they are
not used or charged. Do not
store FLYP for long periods of time
without charging it periodically.
If FLYP is not used or charged
for a long period of time, the
battery may create a hazardous
condition.
Proper handling:
FLYP contains sensitive
components, including a
stainless steel disk with carefully
measured, 4-5 micron holes. Do
not drop, crush, puncture, bend,
heat, incinerate, or apply strong

14 15
•Do not expose the device to
direct sunlight. Keep the device
away from children, pets, pests,
lint and dust. You may render
the device ineffective.
•FLYP is intended for use at
home or a medical facility, such
as a hospital or doctor’s ofce.
Using cords and ports:
NEVER force the USB Wall
Charger into the USB Port.
Check for obstructions in
the USB Port.
shock to the device or its parts.
If you are concerned about
dirtying FLYP™, you can carry it in
the Draw-String Bag.
•NEVER unscrew or open
the Main Unit. Access the
Medication Reservoir by opening
the Reservoir Cover.
•Do not disassemble, repair, or
modify FLYP. You may injure
yourself or render the device
ineffective.
•Keep the device away from
heated surfaces and extreme
heat and cold. Do not leave
FLYP in a car if it will be subjected
to signicant heat or cold.
If the USB Wall Charger and USB
Port don’t join with reasonable
ease, they probably don’t
match. Make sure that you
are using the USB Wall Charger
provided and that you have
positioned it correctly.
Disposing of FLYP
properly:
If, after proper cleaning and
charging, FLYP takes longer
than 10 minutes to deliver either
1 unit-dose of medication or 3
mL of normal saline (0.9%), the
device should be replaced.
Consult “Troubleshooting” on
pages 36-37 to see if corrective
steps can be taken. For
information about the proper
disposal of FLYP, including
other important regulatory
compliance information, see
“Disposal and Recycling” on
page 53.
Intended environment:
The device is intended for
indoor use.

16 17
FEATURES AND BENEFITS
Read this section to learn about
FLYP’s features and benets.
Small and convenient:
FLYP™ ts in your pocket.
There is no separate control unit,
compressor, mask, hose or cup.
And FLYP’s convenient
Draw-String Bag makes it
easy to carry with you.
FLYP is rechargeable with a
USB Wall Charger, like a cell
phone. There are no disposable
batteries. When fully charged,
the battery can power an entire
day’s medical needs, which
should not exceed 3 treatments.
Simple:
FLYP is designed for easy
operation and delivers all
medications approved for use
with general-purpose nebulizers.
Silent:
FLYP operates almost silently,
so it will not disturb children.
Fast and efcient:
FLYP delivers 1 unit-dose in
approximately 7 minutes.
It is designed to shut off
automatically after 10 minutes to
conserve battery life.
If after proper cleaning it takes
longer than 10 minutes to deliver
3 mL of normal saline (0.9%), the
device should be replaced.
Section 3: PRODUCT DESCRIPTION

18 19
NAMES AND FUNCTIONS
OF PARTS
Read this section to learn the
names and functions of the parts.
If any items are missing, contact
the store where you purchased
FLYP. You may also contact
Convexity.
Section 4: FLYP™ AT A GLANCE
RESERVOIR COVER
LATCH
MEDICATION
RESERVOIR
STOPPER
USB COVER
USB PORT
(BEHIND COVER)
MOUTHPIECE
ON/OFF
BUTTON
ON/OFF
INDICATOR
LIGHT
NEBULIZER DISK
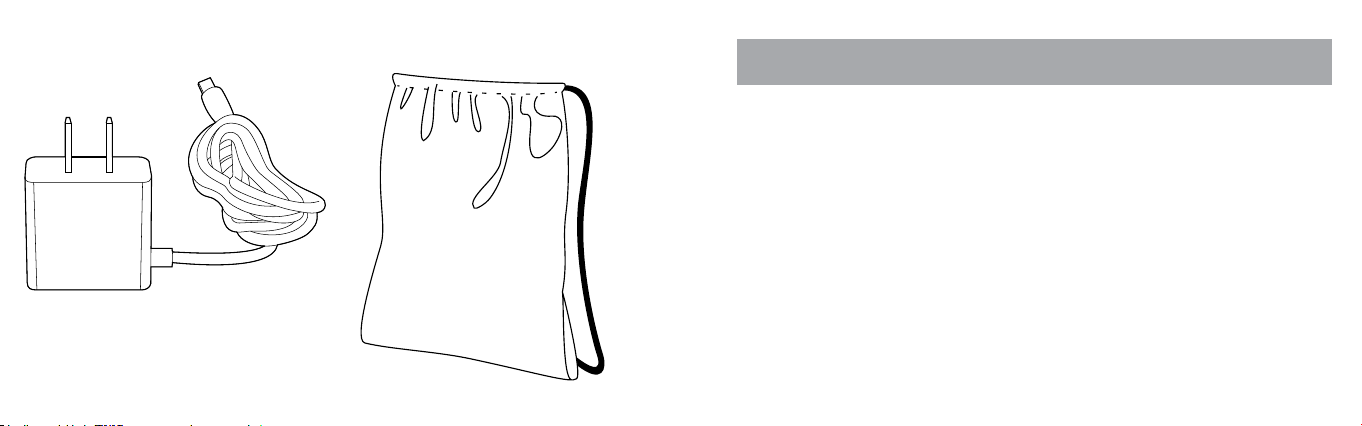
20 21
USB WALL CHARGER DRAW-STRING BAG
Note: the Draw-String Bag is
not intended to protect FLYP™. It is
provided for convenience only.
ABOUT FLYP’S BATTERY
Read this section to learn how to
use FLYP correctly.
About FLYP’s battery:
FLYP is charged through a USB
port, just as many cellular phones
and portable electronic media
devices are charged.
FLYP has an internal battery
that you cannot replace. If
your battery must be replaced,
Section 5: USING FLYP™
contact Convexity Scientic at
P.O. Box 297, Westport, CT 06880,
(203) 557-6254.
For best results, use FLYP fully
charged. Daily users should
charge the device fully between
uses. Do not allow the battery
to remain unused or uncharged
for a long period of time. It may
create a hazardous condition.
Charging FLYP’s battery:

22 23
You can charge FLYP’s battery
by connecting FLYP™ to a wall
outlet using the USB Wall Charger
provided. Only use the charger
provided.
NEVER use the device
while it is charging.
Connection
via wall outlet
To charge the battery with a
wall outlet:
1. Open the USB Cover to reveal
the USB Port.
2. Gently insert the USB Wall
Charger’s Cord into the
USB Port.
3. Insert the USB Wall Charger i
nto a wall outlet.
Understanding battery states:
When FLYP isn’t connected to
a power source, the On-Off
Indicator Light has three
possible states:
When FLYP is connected to a
power source, the On-Off Indicator
Light has two possible states:
Light Signal Status
No Light Go-Neb is Off
Blue Light Go-Neb is On
Blue Light, Blinking Battery is Low
Light Signal Status
Yellow Light, Blinking
Battery is Charging
Yellow Light, Solid
Battery is Fully Charged
Note: Rechargeable batteries don’t
have unlimited life. Battery life and
number of charge cycles vary by use.

24 25
FILLING MEDICATION RESERVOIR
WITH PRESCRIPTION
There is no need to remove the
Medication Reservoir to ll it with
medication.
1. Open the Reservoir Cover,
revealing the Medication Reservoir.
STOPPER
AMPULE
MEDICATION
RESERVOIR
RESERVOIR COVER
STOPPER
STEP 1
2. Unplug the Stopper from the
Medication Reservoir.
STOPPER
AMPULE
MEDICATION
RESERVOIR
RESERVOIR COVER
STOPPER
STEP 2
3. Carefully insert your medication
ampule fully into the
Medication Reservoir. Then
squeeze the entire contents in,
being careful not to spill any.
4. Once the medication is in the
Medication Reservoir, reinsert
the Stopper using your ngers.
Be careful not to touch any
part of the Stopper that may
come in contact with your
medication. Then close the
Reservoir Cover.
STOPPER
AMPULE
MEDICATION
RESERVOIR
RESERVOIR COVER
STOPPER
STOPPER
AMPULE
MEDICATION
RESERVOIR
RESERVOIR COVER
STOPPER
STEP 3 STEP 4

26 27
If you removed the Medication
Reservoir in order to ll it with
medication or to clean it, you
will rst need to reinsert the
Medication Reservoir.
Note: the Medication Reservoir’s
maximum capacity is 5mL.
TURNING FLYP
ON & OFF
Gently raise the Mouthpiece using
your index nger, revealing the
On-Off Button and On-Off
Indicator Light.
To turn FLYP™ on, press the On-Off
Button. The blue On-Off Indicator
Light will light. Visually conrm
than an aerosol mist is owing from
the Mouthpiece’s end.
To turn FLYP off, press the On-Off
Button again. The blue On-Off
Indicator Light will no longer be lit.
FLYP will automatically turn off
after 10 minutes.
MOUTHPIECE
MOUTHPIECE
ON-OFF
INDICATOR
LIGHT
ON-OFF
BUTTON
MOUTHPIECE
MOUTHPIEC
E
ON-OFF
INDICATOR
LIGHT
ON-OFF
BUTTON
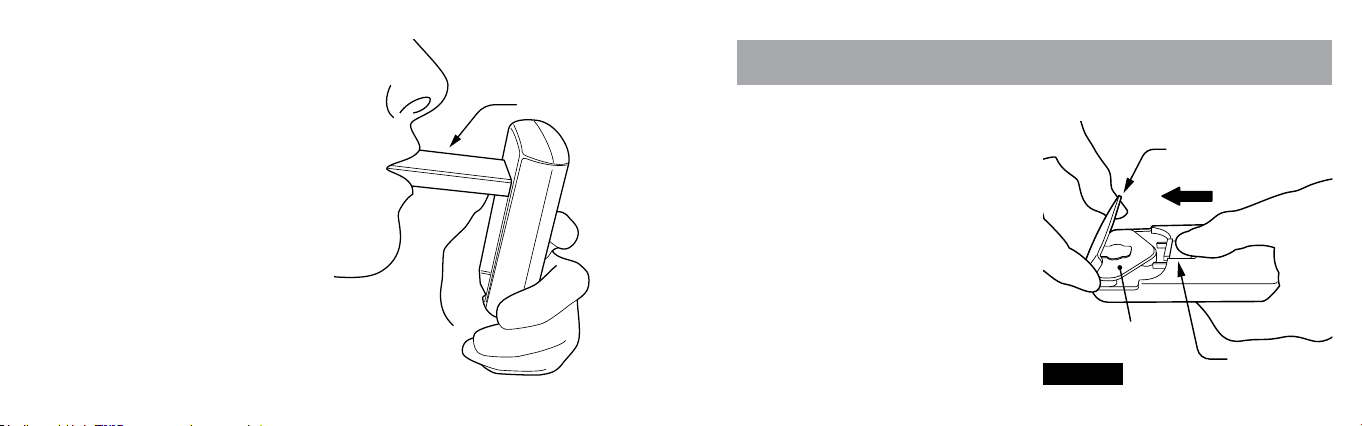
28 29
INHALING PRESCRIBED
MEDICATION
Place the Mouthpiece between
your lips. Inhale and breathe in a
calm manner at a normal rate.
M
OUTHPIECE
Section 6: CLEANING & DISINFECTING
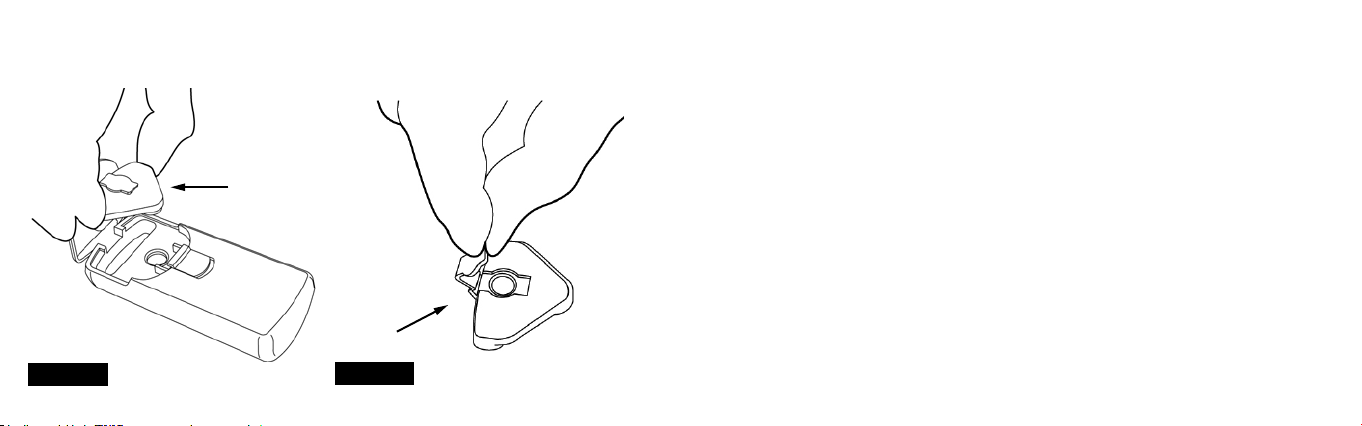
1. Lift the Reservoir Cover.
CLEANING PARTS
Read this section to learn how to
clean FLYP™, both before its rst
use and after each subsequent use.
ALWAYS disconnect and unplug
the USB Wall Charger before
cleaning or disinfecting
Cleaning the Mouthpiece and
Medication Reservoir:
Remove the Mouthpiece by
tugging on it gently. To remove
the Medication Reservoir: STEP 1
STOPPER
LATCH
MEDICATION
RESERVOIR
RESERVOIR COVER
MEDICATION
RESERVOIR
30 31
STEP 2
2. Pull gently on the Medication
Reservoir.
3. Unplug the Stopper from the
Medication Reservoir.
STEP 3
STOPPER
MEDICATION
RESERVOIR
and remove the Mouthpiece.
Prepare a solution of warm water
and mild dishwashing soap. Then
clean the Main Unit by wiping it
with gauze lightly moistened by
the soap solution. Use a clean
gauze moistened with warm
tap water to remove any soap
residue. To dry the Main Unit, wipe
it with dry gauze. Make sure it is
completely dry before reinserting
the Mouthpiece and storing it.
NEVER place the Main Unit or USB
Wall Charger in water or hold them
under running water. The lithium-
ion battery inside the Main Unit
may be dangerous when wet.
Prepare a solution of warm water
and mild liquid dishwashing
soap. Wash the Mouthpiece and
Medication Reservoir in the soap
solution. Rinse the Mouthpiece
and Medication Reservoir under
running tap water. These are the
only parts that can be cleaned
with water. Shake off excess
water and dry on clean, dry towel.
NEVER rinse the Main Unit under
running water.
Cleaning the Main Unit’s exterior:
Prior to cleaning the Main Unit’s
exterior, disconnect and unplug
the USB Wall Charger, make sure
the USB Cover is closed tightly,

32 33
NEVER use window cleaners,
aerosol sprays, solvents, ammonia,
or abrasives to clean FLYP™.
Cleaning the Main Unit’s interior:
1. Fill the Medication Reservoir
with distilled water.
2. Plug the Stopper to seal the
Medication Reservoir.
3. Close the Reservoir Cover.
4. Gently raise the Mouthpiece
with your index nger.
5. Turn FLYP on and allow the
aerosol mist to ow from the
raised Mouthpiece for 1 minute.
6. Unplug the Stopper and Remove
the Medication Reservoir.
7. Shake excess distilled water
from the Medication Reservoir.
8. Let the Medication Reservoir
dry on a clean, dry towel.
9. Let the Main Unit dry on a clean,
dry towel with the Reservoir
Cover open. Allow the Main
Unit’s interior to dry fully before
using it again.
It is important to clean the
Main Unit’s interior to prevent
medication from drying there.
If these steps are not followed, the
4-5 micron holes on the Nebulizer
Disk may become clogged, and it
may not perform properly.
DISINFECTING PARTS
Read this section to learn how
to disinfect FLYP. Although
disinfection is optional, it is
recommended that you do it at
the end of every day.
Disinfecting the Mouthpiece and
Medication Reservoir:
1. Remove the Mouthpiece and
Medication Reservoir.
2. Soak the Mouthpiece and
Medication Reservoir in
70% ethyl alcohol for 10 minutes.
Make sure they are completely
covered with ethyl alcohol.
3. Shake off the excess ethyl
alcohol and rinse the Mouthpiece
and Medication Reservoir under
running tap water.
4. Let the Mouthpiece and
Medication Reservoir dry on a
clean, dry towel.
Disinfecting the Main Unit’s interior:
1. Place FLYP on a at surface and
remove the Mouthpiece.
2. Place a few drops of ethyl
alcohol on the Nebulizer Disk.
Use just enough to cover the Disk
completely. Be careful not to spill
any. Using a dropper may help.

34 35
3. Let the ethyl alcohol sit for 10
minutes. Then shake off the ethyl
alcohol from the Nebulizer Disk.
4. Turn FLYP over. Open the
Reservoir Cover and remove the
Medication Reservoir.
5. Repeat steps 2 and 3 on this side
of the Nebulizer Disk.
6. Place a few drops of distilled
water on the Nebulizer Disk.
Then shake off the distilled
water. Repeat on the other side
of the Nebulizer Disk.
Then remove the ethyl alcohol
from the interior:
1. Reinsert the Medication
Reservoir and Mouthpiece.
2. Fill the Medication Reservoir with
distilled water.
3. Plug the Stopper.
4. Close the Reservoir Cover.
5. Gently raise the Mouthpiece
with your index nger.
6. Turn FLYP™ on and allow the
mist to ow from the raised
Mouthpiece for 10 minutes.
7. Unplug the Stopper and remove
the Medication Reservoir.
8. Shake excess distilled water from
the Medication Reservoir.
9. Let the Medication Reservoir dry
on a clean, dry towel.
10. Let the Main Unit dry on a
clean, dry towel.
11. Allow the Main Unit to dry
fully before using it again.
Disinfecting the Main
Unit’s exterior:
Before disinfecting the Main Unit’s
exterior, make sure the USB Cover
is closed tightly. Disinfect the Main
Unit’s exterior by wiping it with a
clean, dry towel lightly moistened
with ethyl alcohol. Then allow
the Main Unit to sit for 5 minutes.
Moisten a clean, dry towel with
water and wipe the Main Unit to
remove the ethyl alcohol. Allow to
air dry.
NEVER immerse the Main Unit or
USB Wall Charger in ethyl alcohol.
The lithium-ion battery inside the
Main Unit may be dangerous
when wet.
NEVER use window cleaners,
aerosol sprays, solvents, ammonia,
or abrasives to disinfect FLYP.
36 37
TROUBLESHOOTING
Most problems with the device
can be solved by following this
advice.
The following table describes
possible troubles, their causes,
and what corrective action to
take.
Section 7: TROUBLESHOOTING
If these corrective actions do
not return FLYP™ to full working
order, read the next section,
“Support.”
TROUBLE POSSIBLE CAUSE CORRECTIVE ACTION
The
nebulization
rate is
extremely low.
The battery is low. Charge FLYP using a USB
port. Refer to pages 22-23.
An air bubble
has formed in the
medication’s pathway.
Tap the back of the Main
Unit with your index nger
until normal ow resumes.
The Nebulizer Disk has
become clogged with
medication.
Nebulize distilled water for
1 minute. Refer to
pages 29-32.
The On-Off
Indicator
Light is on,
but there is no
mist.
The Medication
Reservoir is not correctly
inserted.
Remove the Medication
Reservoir and install it
correctly.
The Medication
Reservoir is not lled. Fill with medication. Refer to
pages 24-26.
Check that FLYP has
been properly cleaned.
Nebulize distilled water for
1 minute. Refer to pages 29-32.
38 39
You can nd more information about using FLYP™ on our website.
To learn about service and support, and to view tutorials, go to:
www.ConvexityScientic.com/support/FLYP
NOTES:
Section 8: SUPPORT
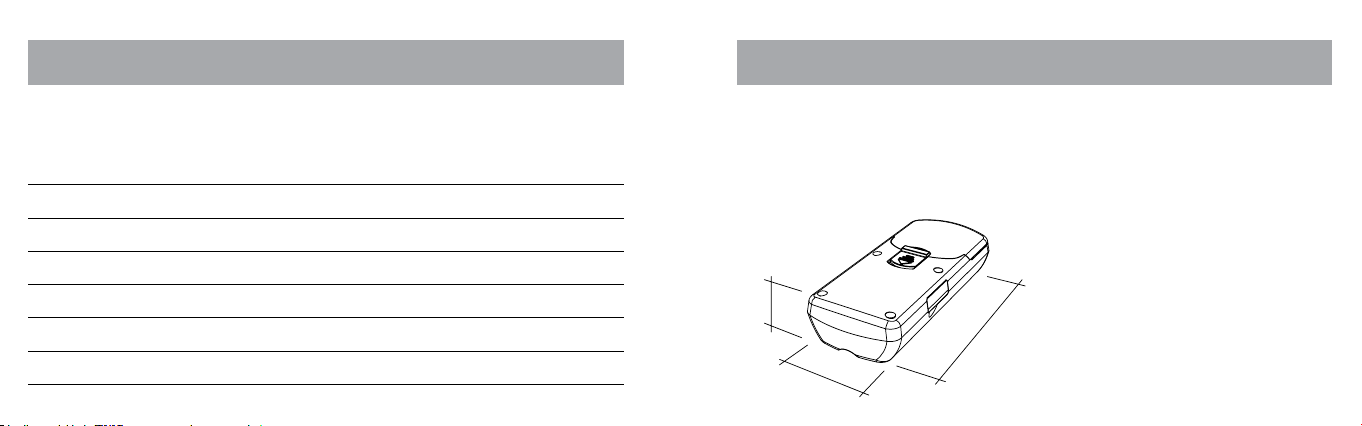
TECHNICAL DATA
Technical Specications:
Length: 119 mm
Width: 54 mm
Depth: 27 mm
Weight: 102 g
(with battery)
Section 9: INFORMATION
LENGTH
WIDTH
DEPTH
SPECIFICATIONS
Indications for use:
The FLYP™ nebulizer, for use by
adolescent and adult patients, is
intended to aerosolize healthcare
provider-prescribed solutions
for inhalation that are approved
for use with a general-purpose
nebulizer. FLYP is intended for
use at home or a medical
facility, such as a hospital
or doctor’s ofce.
Other manuals for pe1200m
1
Table of contents
Other flyp Respiratory Product manuals




















