EN-1
Safety Information
Read the following Safety Information thoroughly before using
the device.
• Use this device ONLY for the intended use described in this
manual.
• Do NOT use accessories which are not specied by the
manufacturer.
• Do NOT use the device if it is not working properly or
damaged.
• This device does NOT serve as a cure for any symptoms or
diseases. The data measured is for reference only. Always
consult your doctor to have the results interpreted.
• The blood glucose test strip can NOT be used for the
testing of newborns.
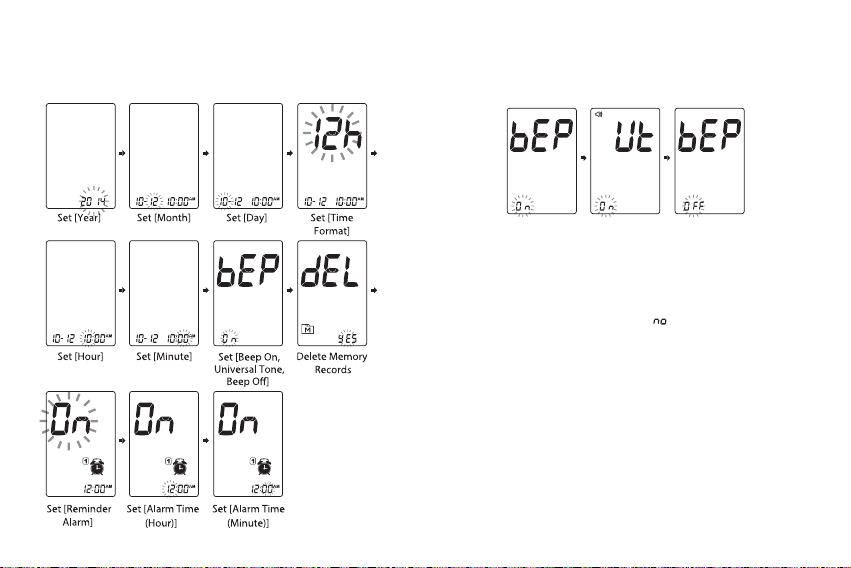
• Before using this device to test blood glucose, read all
instructions thoroughly and practice the test. Carry out all
the quality control checks as directed.
• Keep the device and testing supplies away from young
children. Small items such as the battery cover, batteries,
test strips, lancets and vial caps are choking hazards.
• The use of this instrument in a dry environment, especially
if synthetic materials are present (synthetic clothing,
carpets etc.) may cause damaging static discharges that
may cause erroneous results.
• Do NOT use this instrument in close proximity to sources
of strong electromagnetic radiation, as these may interfere
with the correct operation.
• Proper maintenance as well as timely calibration of the
device together with the control solution is essential in
ensuring the longevity of your device. If you are concerned
about the accuracy of the measurement, please contact
the place of purchase or customer service representative
for assistance.
KEEP THESE INSTRUCTIONS IN A SAFE PLACE
Important Information
• Severe dehydration and excessive water loss may cause
readings which are lower than actual values. If you believe
you are suering from severe dehydration, consult a
healthcare professional immediately.
• If your blood glucose results are lower or higher than usual,
and you do not have symptoms of illness, rst repeat the
test. If you have symptoms or continue to get results higher
or lower than usual, follow the treatment advice of your
healthcare professional.
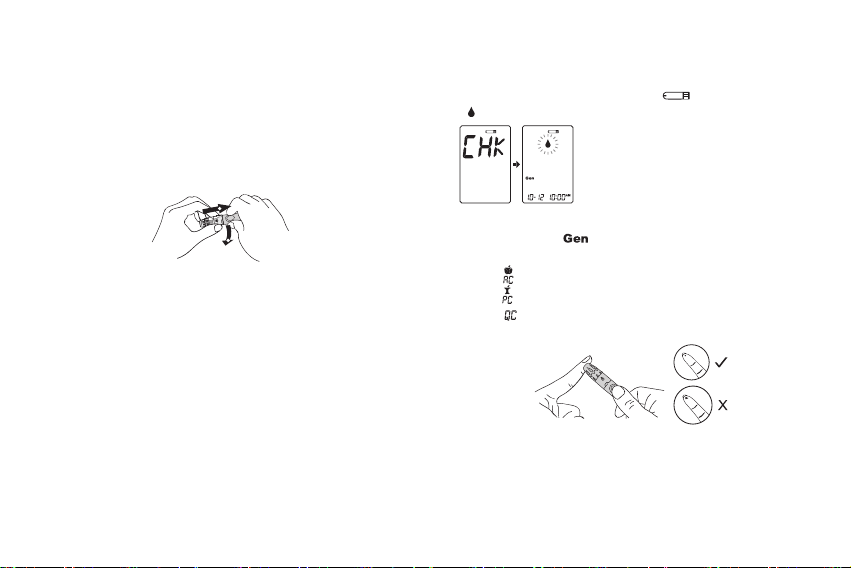
• Use only fresh whole blood sample to test your blood
glucose. Using other substances will lead to incorrect
results.
• If you are experiencing symptoms that are inconsistent
with your blood glucose test results and you have followed
all instructions described in this owner’s manual, call your
healthcare professional.
• We do not recommend using this product on severely