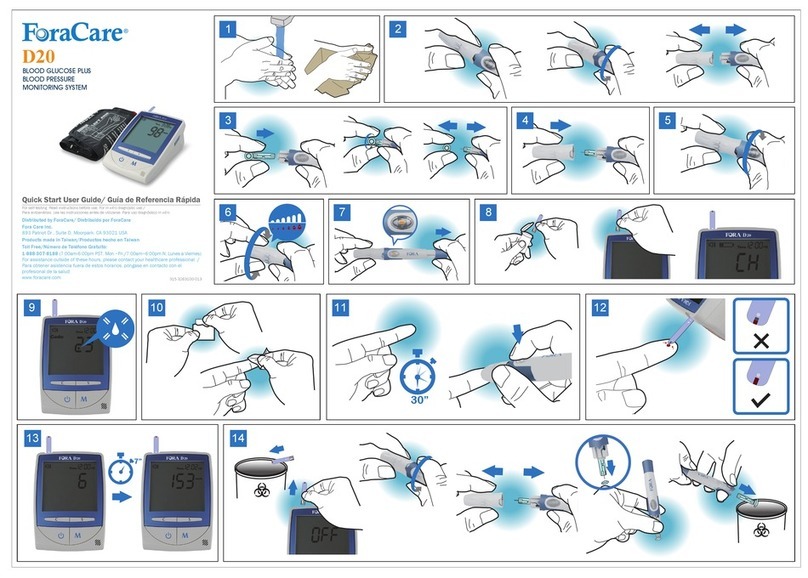
EN-8
For P30 Plus and P30 Plus BT
Press to review the average and general memory recall mode.
Note:
• Day-time average ( ) is the average of the measurements taken
during 4:00 A.M. to 11:59 A.M.
• Night-time average ( ) is the average of the measurements taken
during 6:00 P.M. to 11:59 P.M.
• Press to exit the memory mode or leave it without any action for
3 minutes. The device will turn o automatically.
• If using the device for the rst time, the “---” icon appears when you
recall the test results or review the average result. It indicates that
there is no test result stored in the memory.
Classication of Blood Pressure
Human blood pressure naturally increases after reaching middle
age. This symptom is a result of continuous ageing of the blood
vessels. Further causes include diabetes, lack of exercise and
cholesterol (LDL) adhering to the blood vessels. Rising blood
pressure accelerates hardening of the arteries, and the body
becomes more susceptible to apoplexy and coronary infarction.
This device does NOT serve as a cure for any symptoms
or diseases.
The data measured is for reference only. Always consult
your physician to have the results interpreted.
Denitions and Classication of blood pressure levels according
to 2007 ESH-ESC Practice Guidelines for the Management of
Arterial Hypertension:
Category Systolic Diastolic
Optimal < 120 mmHg and < 80 mmHg
Normal 120 –129 mmHg and/or 80–84 mmHg
High normal 130 –139 mmHg and/or 85–89 mmHg
Grade 1 hypertension 140 –159 mmHg and/or 90– 99 mmHg
Grade 2 hypertension 160 –179 mmHg and/or 100–109 mmHg
Grade 3 hypertension ≥ 180 mmHg and/or ≥ 110 mmHg
Isolated systolic
hypertension ≥ 140 mmHg and < 90 mmHg
Isolated systolic hypertension should be graded (1, 2, 3) according
to systolic blood pressure values in the ranges indicated, provided
that diastolic values are < 90mmHg.
Source: The European Society of Hypertension and European
Society of Cardiology Task Force Members. 2007 ESH-ESC Practice
Guidelines for the Management of Arterial Hypertension. J
Hypertens 2007; 25: 1751-1762.
Maintenance
Changing Battery
When the battery is low, one of the following
screen will appear:
• the “ “ icon appears with display messages
: This indicates the device is functional and
the result remains accurate, but it is time to
change the batteries.