foras OXY300 User manual

Oxygen Concentrator User Manual
FORAS
OXYGEN CONCENTRATOR
USER MANUAL
MODEL : OXY 00 – OXY500
CAUTION: Please read this manual before using.
1 / 18

Oxygen Concentrator User Manual
Table of Concents Page No
Cover 1
Index 2
Introduction, Cautions and General Precautions
Indications, Contraindications and Adverse Effects 4
Patient Population and Accessories of Oxygen Concentrator 5
Back View, Screen View and To-Do Before Operating Device 6
Humidifier Bottle Usage 7
Nasal Cannula Usage, Oxygen Concentrator Turn On/Off 8
Maintenance and Controls 9
Calibration and Cleaning 10
Alarms, Technical Specifications 11
Environmental Conditions and Troubleshooting Tables 12
Symbols Explanation, 1
Residual Risks 14
Related Directives, Electromagnetic Compatibility 15
Compliance with Standarts, 60601 Classification and Software Information 17
Manufacturer Information, Notified Body Information 18
2 / 18

Oxygen Concentrator User Manual
INTRODUCTION
This manual will introduce OXY 00 and OXY500 Oxygen Concentrators. Read this
Read and understand the user manual before operating the device. Important safety
precautions are described in this manual. Please pay attention to all information. Consult
your doctor in any adverse situation.
CAUTION : For your safety, use in accordance with the advice of specialist physcian.
WARNING : Do not smoke near the person who receives oxygen therapy while the
oxygen concentrator is operating. Oxygen causes rapid combustion. Keep the oxygen
concentrator at least 2 meters away from sparkable objects and bare sources of flame.
CAUTIONS AND GENERAL PRECAUTIONS
•It does not require special training and skills to use the device. Professional or first-
time users will have met the minimum terms of usage conditions when reading the
user manual.
•In the event of a power cut, failure of the Oxygen Concentrator or insufficient
oxygen supply to the patient, it is obligatory to carry a spare oxygen provider or
oxygen cylinder with you. This is your responsibility. Foras Medical does not accept
any responsibility for customers who do not comply with the manufacturer's
instructions.
•Foras Medical does not accept any problems that may arise from materials used
other than the recommended accessories. Do not modify the device and do not
allow any company except Foras Medical to intervene device during the warranty
period. In such cases, the device will be excluded from warranty.
•Do not carry the device when there is water in water tank or take precautions to
avoid overturning. If water leaks into the device, the device is out of warranty.
•Do not smoke in the room/environment where the device operates.
•Since the oxygen is a flammable gas, care should be taken against risk of ignition.
Keep far away.
•Keep the device away from humid (more than 95% humidity) environment. Do not
expose to water and sediment. Do not operate in the rain, it may cause electric
shock.
•Do not use the device in an excessive dusty and smoky environment. Check the
filter frequently. When the device is operating in a dusty environment, it will cause
the oxygen level to decrease due to the blockage of the air inlet filter.
•The device may show low performance at high temperatures (over 40 ° C). Do not
expose the device to direct sunlight. Use the device in a shade and airy place.
•Do not keep petroleum-based products (oil, petrol grease, etc.), flammable and
sparkler materials (thinner, alcohol, cologne etc.) near the device. Care should be
taken against the risk of burning the device
•It is not used on these patients as there is no clinical trial for its use in the treatment
of pregnant, lactating women and young children.
•Device has wheels underneath for easy movement. Open the brakes on the wheel
while moving on the ground. Otherwise, it may cause the device to overturn.
•Carrying handle should be used for easy transportation of the device.
•Do not place the device on tables, coffee tables, etc. It should be noted that the
device can be toppled because it emits vibration while operating.
3 / 18

Oxygen Concentrator User Manual
•If you feel sick or uncomfortable while using the device, consult your physician.
•The doctor or caregiver should take protective measures against the risk of cross-
infection if the patient has an infection. At the same time, no one at the risk of
infection should have contact with the patient.
•Operate the device in an upright position. The device should not be operated
horizantally.
•When the device has completed its life, it should not be disposed with household
waste, it should be disposed with medical waste.
•The device does not ope ate at full pe fo mance fo 24 hou s. The efo e, the it
should be closed at least 2 hou s in a daily. Fo such cases, an oxygen
cylinde and a backup oxygen p ovide should be available.
•Keep the ight, left and back of the device at least 50 cm away f om the wall.
Keep away f om heating devices such as stove and heate .
•It is not suitable to use with MR devices.
WHY IS OXYGEN THERAPY RECOMMENDED ?
Many people suffer from heart, lung and other respiratory diseases. Most of these
people benefit from additional oxygen therapy. Our body needs constant oxygen to
function properly. Your doctor prescribes for supplemental oxygen because you can not
get enough oxygen in the ambient air. This additional oxygen will meet your body's oxygen
needs.
Supplemental oxygen is not addictive. Your doctor has prescribed a specific oxygen
flow to enhance symptoms such as headache, drowsiness, fatigue or increased irritability.
Contact your doctor if these symptoms persist after starting treatment.
Today, oxygen concentators are reliable, efficient and convenient source of oxygen.
Oxygen concentrator separates the oxygen from the air in the environment, thus the
concentrator filters the oxygen in a tank and provides high purity supplemental oxygen to
the patient from the oxygen output.
INDICATIONS AND INTENDED USE
It is used for chronic lung disease (Chronic Obstructive Pulmonary Disease -
COPD), on the treatment of patients who need supplemental oxygen and in cases where
the patients need pure oxygen due to heart disease.
CONTRAINDICATIONS AND ADVERSE EFFECTS
Side effects and contraindications that may occur in use of the device contrary to
the user manual;
Other than the recommended use by physician, oxygen therapy of the device may
be harmful.
In the use of patients with vision/hearing loss, problems may occur if the instruction
is not followed.
The oxygen concentrator should not be used with other respiratory apparatus other
than the recommended devices. Low performance can be observed use with other
devices. As a result of this, the patient and the device may be damaged.
If the device is not used for its intended use, it may harm the patient.
If it is not used as recommended in the instruction, it may take risk because it
contains oxygen.
Device can be damaged as a result of water leak in case of using humidifier.
4 / 18

Oxygen Concentrator User Manual
Smoking in the environment where the device is located and to keep flammable
materials near the device may be dangerous.
If the device is used above the moisture specified in the instructions, oxygen
treatment will not be possible because the columns will be damaged.
If the device is used above the temperature which specified in the instruction, it
should be noted that there will be low performance on the device.
It is objectionable to approach the device with flammable and explosive materials.
PATIENT POPULATION
It ise used in chronic lung system disease, other diseases due to lack of oxygen and
for patients that need supplemental oxygen who are prescribed by doctor.It is not used in
following patients as there is no clinical study has been conducted on the use of pregnant
and breastfeeding women, kid.
ACCESSORIES OF OXYGEN CONCENTRATOR
The following parts are included in standart package content of Oxygen
Concentrator.
•Oxygen concentrator main unit
•Humidifier bottle
•Humidifier bottle connection tube
•Nasal cannula
•User manual
F ont View
(Figu e 1)
Back View
5 / 18
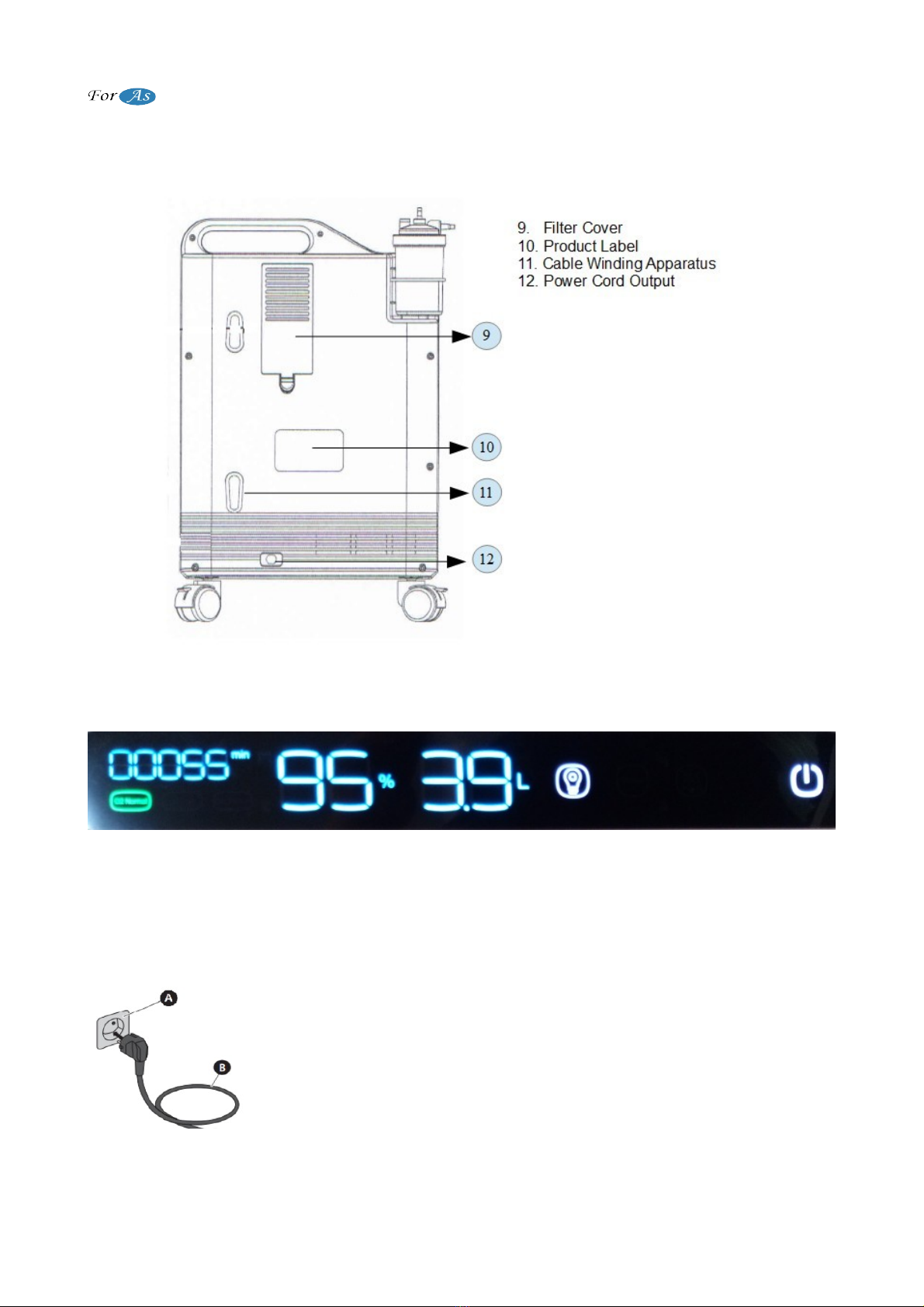
Oxygen Concentrator User Manual
(Figu e 2)
Display View
(Figu e 3)
Note: When there is electricty, the icon on the power indicator is green colored. It is red
colored when O2 Low and Call Service icons appeared.
BEFORE OPERATE THE OXYGEN CONCENTRATOR
Afte emoving the device f om its box, the inst uctions fo use must be ead befo e
ope ating device.
1. After the device is removed from box, it is checked whether there is
physical damage. Then the electrical cord of the device is plugged into
the electrical outlet in the room.
A. Mains Electricity (Outlet)
B. Concentrator Power Cord
(Figu e 4)
6 / 18
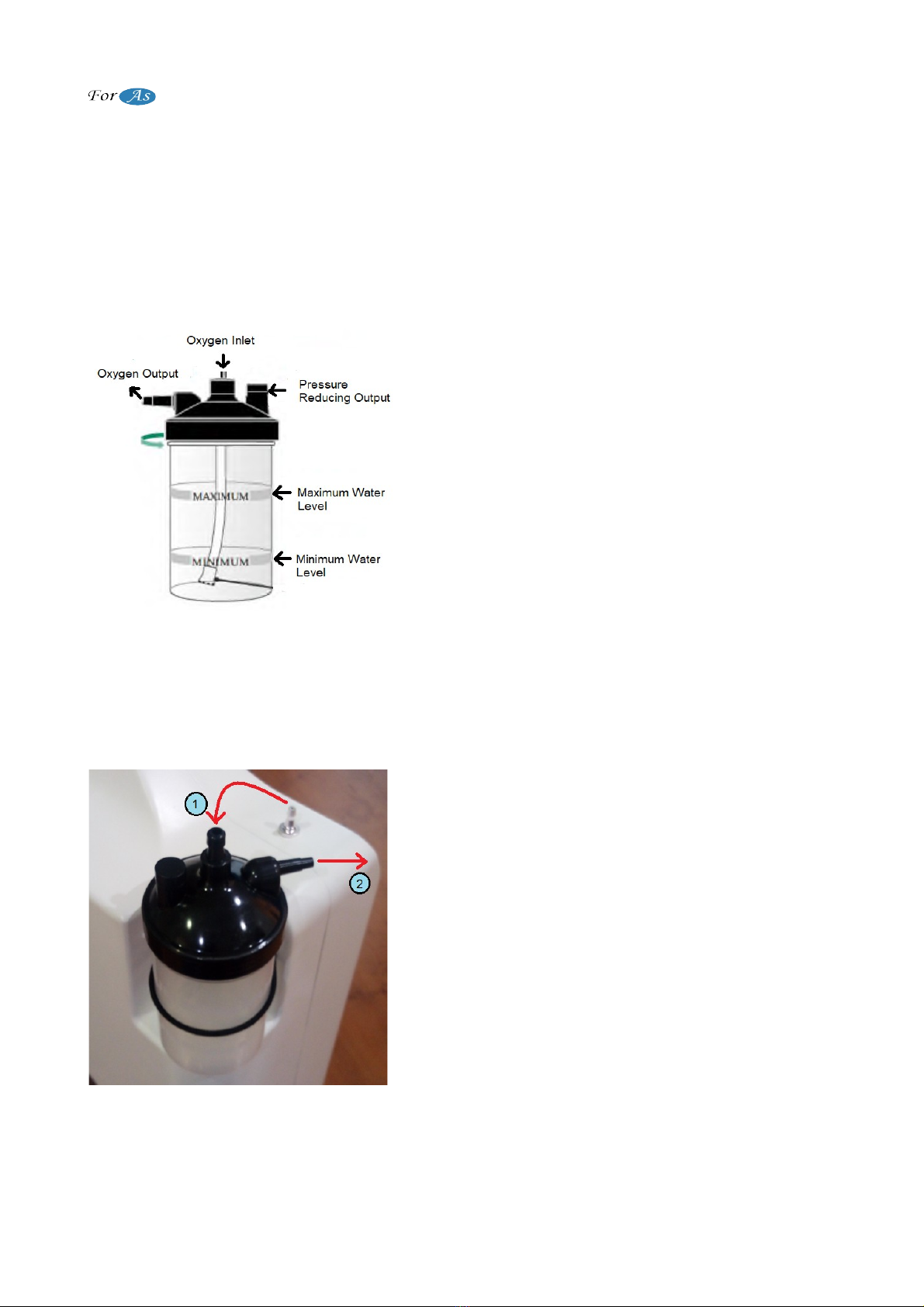
Oxygen Concentrator User Manual
Note: Do not attach any other device to the outlet where the oxygen concentrator is
connected.
2. Before operating the device always make sure that the air filter is installed and
clean.
. Attach the recommended Oxygen Concentrator accessories (Humidifier Bottle and
Cannula) to the device.
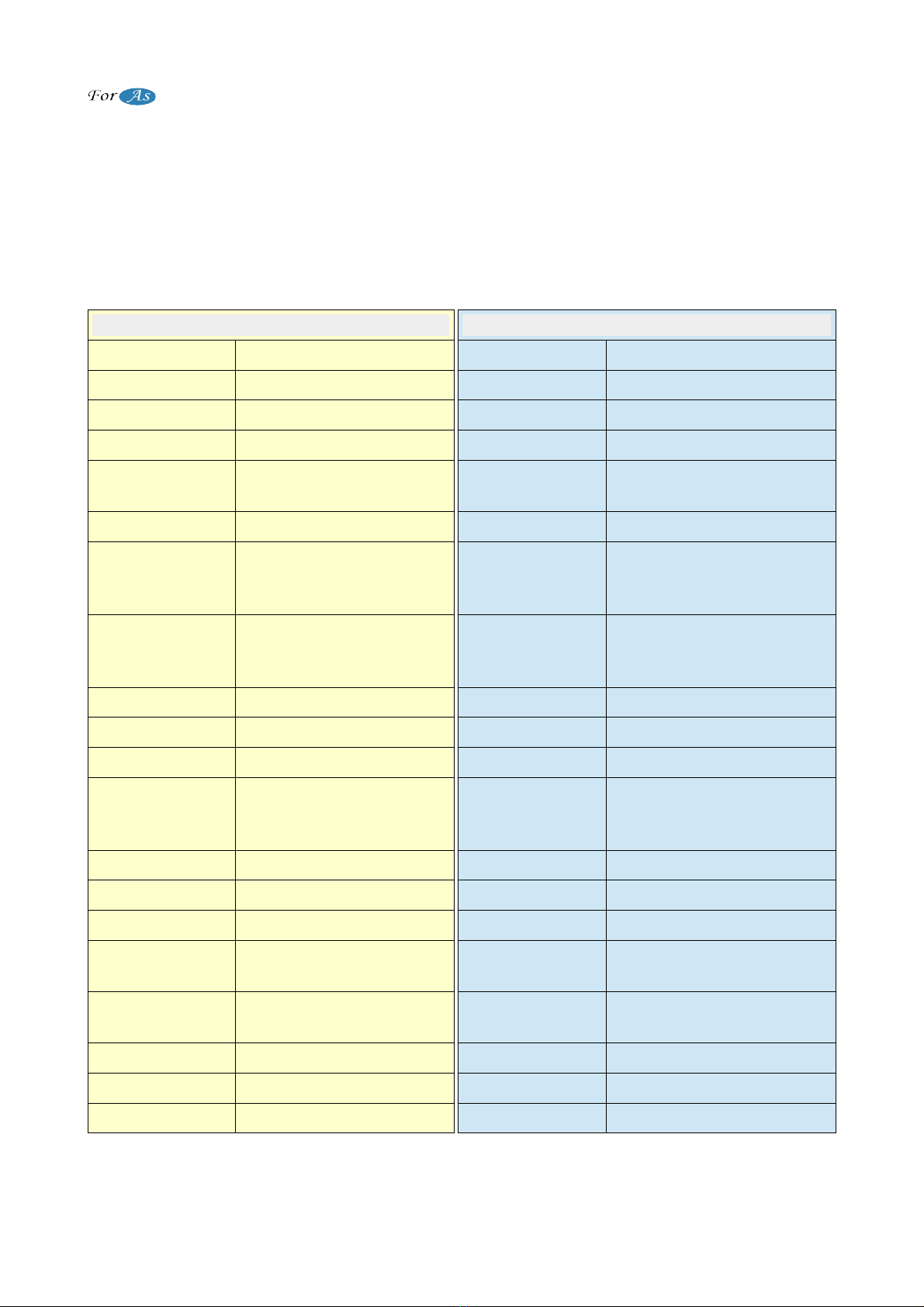
Humidifie Bottle Usage
a.) Remove the humidifier bottle cover by turning
it in the direction of narrow.
b.) Put water to humidifier bottle until Maximum
level. Do not exceed maximum level (As
manufacturer, we recommend you to put water
between maximum-minimum levels and to
replenish the water every day.) Put boiled and
chilled or bottled water to the humidifier bottle.
(Do not use tap water)
c.) Close the lid and replace the humidifier bottle
on oncentrator. When installing the cover, make
sure it is properly installed. Otherwise it could
leak oxygen through the hatch.
d.) Connect the oxygen hose that comes from
oxygen concentrator to the oxygen inlet.
e.) Connect the cannula to the oxygen outlet.
(Figu e 5) f.) Make sure there is no calcification or
sediment in the humidifier bottle.
Humidifie Bottle Tube Connection
1. Interconnecting tube is connects from oxygen outlet
of device to the place on the humidifier bottle that
shown in Figure 6.
2. Nasal cannula connects to oxygen outlet.
NOTE: Care should be taken to avoid calcification and
blockage of the hose tip in the humidifier bottle. If the
humidifier bottle will not be used, the cannula must be
connected directly to oxygen outlet.
If the humidifier bottel is not to used, the cannula
should be connected directly to the oxygen outlet. The
image of the humidifier bottle is its representation, it
can differs from the original. Humidifier bottle should
be changed once in a month.
(Figu e 6)
7 / 18

Oxygen Concentrator User Manual
Nasal Cannula Usage
It is a tubing system made of from plastic-derived biocompatible material that placed in
the nostrils of the patient who use the device and it provides oxygen delivery to the
patient.This tubing system, does not prevent to patient's deating/drinking activities. Patient
can continue his/her daily life with nasal cannula. In case of any tear, break and wear out,
do not use the cannula and replace it with new one.
Cannula should be connected to the oxygen outlet of the humidifier bottle of the
Oxygen Concentrator. The recommended cannula length is 2 meters.
If there is a risk of infection on the patient, protective equipment should be used
when applying nasal cannula to the patient. Precautions should be taken against the
risk of cross-infection from the patient or the risk of infection to the patient. Cannula
removed from the patient must be disposed of in accordance with medical waste
procedure.
CAUTION !: In case of obstruction of cannula, keep the tube away from children and
animals.
Figure 7 shows the general view of the
cannula and Figure shows the patient
connection form of the cannula.
Make sure that the cannula used is not
fold, broken and torsion. Otherwise, patient
might have a problem due to oxygen
therapy can not be applied.
(Figu e 7) (Figu e 8)
Note: Use the cannula or mask recommended by your doctor. It is recommended to
replace once in a month.
TURN ON / OFF FOR OXYGEN CONCENTRATOR
Do not operate the device without taking the necessary controls and precautions
before operating the device.
CAUTION : Do not smoke near the device, do not let smokers close to device.
After plugging the power cord of the device into the outlet, start the
device by pressing "I" shaped position as showed in Figure 9. The
display lights turn on when the button is pressed. Then, working time of
the device appears and it starts operating.
Contact the manufacturer if the device does not work or any
abnormalities are detected. Starting of device takes 1 second when the
power button is pressed.
(Figu e 9)
8 / 18

Oxygen Concentrator User Manual
Flowmete
After operating the device, desired setting should be made by
adjustment knob shown in Figure 10. When the button is turned to
the "+" side, the flow increases. When the button is turned to the "-"
side the flow decreases. If there is no change in the flow of the
display when the button is turned, contact technical service.
(Figu e 10)
Flow increases when the adjustment knob is turned to the "+" side. Flow decreases when
the adjustment knob is turned to the other side.
When the flowmeter is turned clockwise, it decreases and stops at the last point. It
rises when reversed.
CAUTION ! : Use the flowmeter to adjust the oxygen recommended by your doctor.
Otherwise, do not change the oxygen flowmeter setting by yourself.
Not: You may want to set the oxygen flowmeter for you where you bought the device.
Device can be turned off by pressing "0" as shown in Figure 11.
When the button is presses, it turns on immediately.
(Figu e 11)
MAINTENANCE AND CONTROLS
Grease oil, machine oil and any kind of oil like this should not be used in the device
in any way.
CAUTION !: Keep the device off while performing general controls and maintenance.
Make sure that the cabinet of device is not opened by yourself or unauthorized
persons. Do not use it in any way in case there is an opening in cabinet. The cabin
must be opened only by an authorized personnel. Avoid liquid contact with the device.
It is ecommended that the device se viced once in eve y 12 months. The
pe fo mance of the device depends on its p ope ope ating.
9 / 18

Oxygen Concentrator User Manual
Oxygen Humidifie Bottle Checkings
If your doctor recommends using the device with humidifier;
•Check the humidifier bottle periodically, as sediment and lime will form in the water
tank during extended use.
•It is recommended to change the humidifier bottle once a month.
•Add drinking water (bottled water) or boiled water daily. Do not use tap water.
•Do not put more / less water than the indicated level.
•Make sure the lid is properly closed after adding water.
Cannula Checking
Make sure that the cannula used is not broken and not torsion. We recommend that
you change the cannula at the latest in 1 month depending on the intensity of use.
Filte Checking
Under the normal use conditions, it is recommended that the bacteria filter of device to be
changed once in a month. However, this time gets shorter using in very dusty environment.
The filter should be checked and changed frequently. This responsibility belongs to patient.
The device and the patient may be damaged by the blockage of the filter. If the device is
damaged due to failure to filter not being replaced on time, the device will be out of
warranty even it is under warranty term.
Changing the filter;
•Open the filter cover on the back of the device.
•Remove the filter and replace it. (Never try to clean the filter)
The sponge filter on the device (if any) should be checked frequently and removed
when it is contaminated. After washing with warm water, it should be dried and used again.
Worn filters should not be used.
The proper operation of the device is not only depends on device. Accessories are
very important. Checkings should be carried out as described above. Otherwise,
performance will decrease.
CALIBRATION
Operation of the device at the desired performance can be achieved by following
the instructions. In normal use, it is sufficient to calibrate the device once in 12 months. If it
is suspected that there is a decrease in the oxygen rate of the device, it should be verified
by measuring it with a calibrated oxygen analyzer.
CLEANING
Do not use petroleum based and solvent based products when cleaning the cabinet
of device. Clean the outer cabinet of device with alcohol through cloth. Thanks by this,
there will be no residue. Unplug the power cord from outlet while cleaning.
10 / 18
Oxygen Concentrator User Manual
ALARMS
O² Low : When that audible and visual alarm emerges on device, it indicates that
the oxygen level decreases below %82..
Call Se vice : The device gives an audible and visual alarm in the event of a power
failure and when the flowmeter is 0 (zero).
TECHNICAL SPECIFICATIONS
OXY 00 OXY500
Oxygen Level 1- LPM Oxygen Level 1-5 LPM
Pressure 8,5 psi (58.6 kpa) Pressure 8,5 psi (58.6 kpa)
Watt 00W Watt 00W
Electrical Spec. 2 0V, 50Hz, 1.8A(max) Electrical Spec. 2 0V, 50Hz, 1.8A(max)
Oxygen
Percentage
1~ LPM 94%± % Oxygen
Percentage
1~5 LPM 94%± %
Fuse 15A Fuse 15A
Av. O² Purity 1 lt/min. : %94± %
2 lt/min. : %94± %
lt/min. : %94± %
Av. O² Purity 2 lt/min. : %94± %
4 lt/min. : %9 ± %
5 lt/min. : %92± %
Operating
Conditions
Temp. :10ºC / 40ºC
Humidity : % 0 / %70
Pressure : 50/106kpa
Operating
Conditions
Temp. :10ºC / 40ºC
Humidity : % 0 / %70
Pressure : 50/106kpa
Weight 14kg Weight 15kg
Sound Level <48dbA Sound Level <48dbA
Dimensions 400* 00*510mm Dimensions 400* 00*510mm
Storage
Conditions
Temp. :-20ºC/ 50ºC
Humidity : %0 / %95
Pressure : 50/106kpa
Storage
Conditions
Temp. :-20ºC / 50ºC
Humidity : %0 / %95
Pressure : 50/106kpa
O² Capacity Liter O² Capacity 5 Liter
Model Diff. Continuously lt Model Diff. Continuously 5 lt
Working System Producing electric withO² Working System Producing electric withO²
Class/Protection
type
II / BF Class/Protection
type
II / BF
Expected
Operating Life
10 years Expected
Operating Life
10 years
Warranty 2 years Warranty 2 years
IP Value IP21 IP Value IP21
Alarm Available Alarm Available
11 / 18
Oxygen Concentrator User Manual
ENVIRONMENTAL CONDITIONS
•Do not use oxygen concentrator devices in any toxic environment.
•Do not use oxygen concentrator devices in environmental conditions where
explosive and chemical gases or other flammable anaesthetic agents are present.
Keep fire away.
•Do not use under rain, direct sunlight, in extremely humid-dusty environment or in
smoking areas.
•Storage Conditions: Temperature -20ºC / 50 ºC, Humidity %0 - %95, Pressure
50kPa/106kPa
•Operating Conditions: Temperature 10ºC / 40 ºC, Humidity % 0 - %70, Pressure
50kPa/106kPa
•The device performance will decrease in case of storing out of abovementioned
temperature, humidity and pressure limits.
TROUBLESHOOTING TABLES
T ouble Solution
Device does not deliver
oxygen
* Make sure that the power cord of device is plugged to
outlet.
* Make sure that device is operating.
* Make sure the flowmeter is set as required.
* Check the connections of nasal cannula.
* Make sure the lid of humidifier bottle is closed properly.
* Make sure the tube inside humidifier bottle is not
blocked.
* Contact technical service
Device gets overheat
* Make sure that device is not close to wall.
* Make sure the filters are not dirty.
* Pay attention that the environment is not too hot where
the device is used.
* Pay attention to the ventilation of the environment where
the device is used.
* Contact technical service.
Device emerges O² Low
alarm
* Ventilate the environment where device is located.
* Contact technical service.
Device shows Call Service
alarm
* Make sure that electrical power is supplied to the device.
* Make sure flowmeter setting is not at 0 (zero) or over 5.
* Contact technical service.
Device operates noisily * Operate the device on flat surface.
* Contact technical service.
No air flow on device
* Put your finger to the oxygen outlet of the device.
* Check the flow showed on display.
* Contact technical service.
12 / 18
Oxygen Concentrator User Manual
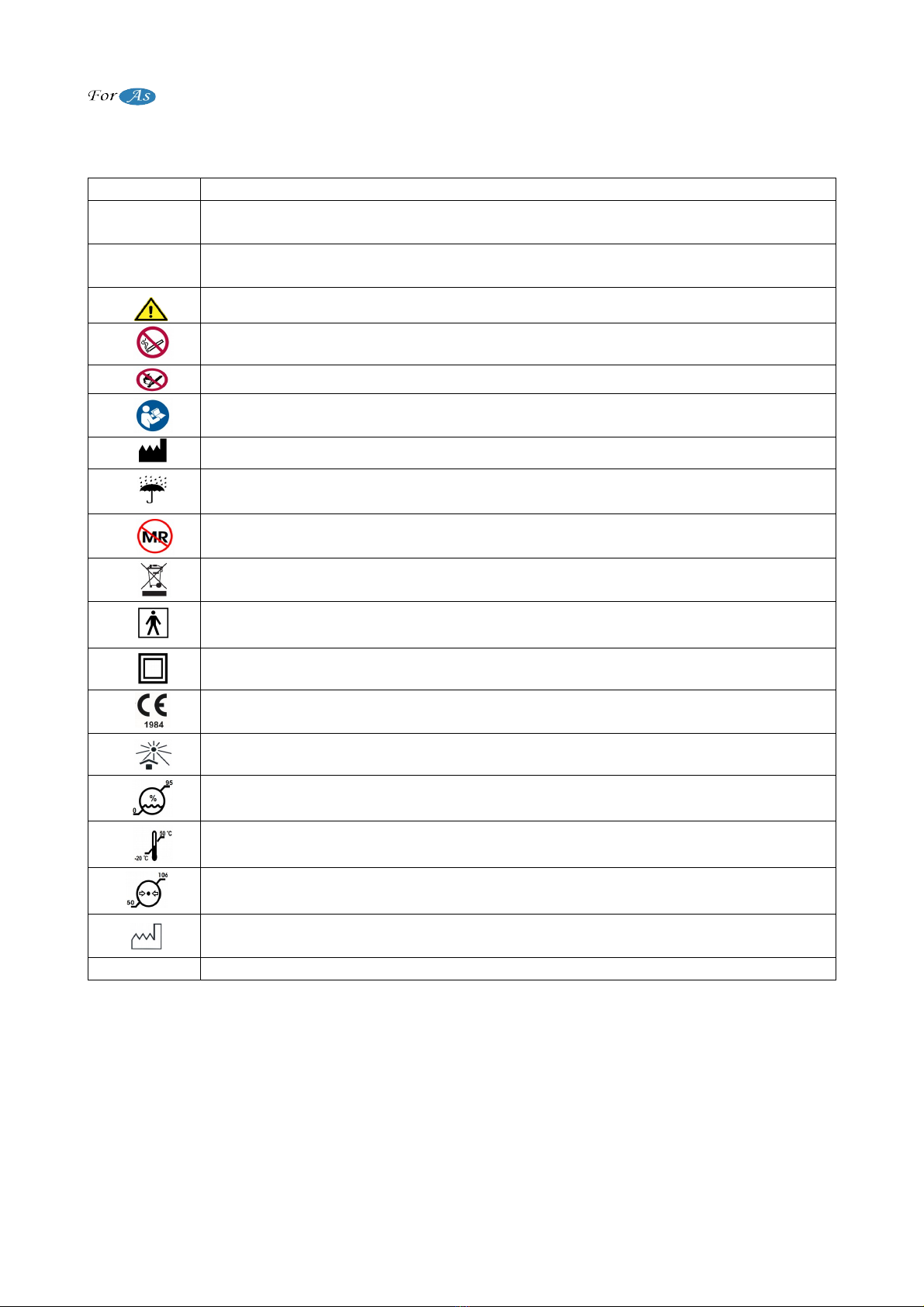
SYMBOLS EXPLANATION
Symbols Explanation
WARNING
“WARNING”, indicates the situations that safety of patient may be in
danger.
CAUTION
“CAUTION”, indicates that precautions should be taken and maintenance
instructions should be followed
Check the user manual for instructions
Do not smoke while using device
Keep fire away
Chek the user manual for instructions
Manufacturer
Keep dry
It is not suitable for use with MR device.
Do not dispose in street garbage
BF type
Clas II Equipment
CE Mark and Notified Body Identification Number
Do not expose to sunlight
Humidity Limits
Temperature Limits
Pressure Limits
Production Date
IP21 IP Rate
13 / 18

Oxygen Concentrator User Manual
RESIDUAL RISKS
Potential Risk Residual Risk
Application of Line Voltage Usage without reading user manual
Voltage Leakage To User Failure to perform leakage tests and final checks
Electric Shock Failure to perform leakage tests and final checks
Magnetic Field/Radiation EMC test values are not declared in the manual
Ionized Radiation Energy Usage outside declared values
Non-Ionising Radiation Usage outside declared values
Heat Given By Device Intervention to the device out of service
Falling Inappropriate carrying of device
High Sound Emission Improper assemble and production
Titration Improper assemble and production
Stored Energy Sensor failure or manual adjustment
Acustic Energy Usage outside declared values
Not given O2 due to cannula Failure to comply with intended use
Gases formed by chemical wastes Non-compliance with given trainings
Medical Gases Non-compliance with given trainings
Additives Failure to comply with critical raw material instruction
Residue on the device Failure to read user manual
Bacteria, virus and other agents Non-compliance with trainings and instructions
Again or Cross Infection Usage of non-informed persons
Biocompatibility Product use without CE certificate
Contaminants Not paying attention to manual
Function error Failure of final checkings and malfunction of sensors
Model Difference Patient's failure to use appropriate device
Error due to lack of attention Not paying attention to manual
Causing memory weakness Failure to use appropriate device for report and
prescription
Damage due to misuse No training and not reading of manual
Lack of knowledge Failure to read the manual and rejection of training
Frequent violations Not reading the manual and wrong habits
Improper use of device acc. No training and not reading of manual
Error before device operation Failure to read the manual and not receive training
Long-term use errors Failure to read the manual and not receive training
Lack of side eff. and warnings Not paying attention to product labels and symbols
Deflagration Not reading the manual and not paying attention to
labels
Usage in extremely humid envr. Not paying attention to product labels
Usage in a dusty environment Use of untrained persons
Using on pregnants and children Usage of untrained persons and not reading manual
Water leakage into device Usage of untrained persons and not reading manual
Problems due to critical material Failure to follow instructions
Assemble Error Staff Carelessness
Plastic manufacturing failure Failure to act in accordance with the list
Adverse events Lack of knowing responsibilities and authorities
Recalls Lack of knowing responsibilities and authorities
Untraceability Hazırlanan form ve kayıtların tutulmaması
Usage out of intended purpose Failure to fill the inspection and test forms
14 / 18

Oxygen Concentrator User Manual
Contunation of identified prolems Failure to perform corrective actions
Packaging Mistake Failure to comply with procedure
Labelling Mistake Failure to comply with instructions
Lack of operational instructions Failure to read user manual
Disposal of the device to trash Usage of untrained persons and not reading manual
Lack of warnings Not reading the manual, not paying attention to the
label
Software Failure Failure to perform final control tests
Processor Failure Failure of performing input checkings
Failure of the device Failure to act in acordance with user manual
Sensor Errors Failure to perform final checks
Device error after service Failure to act in accordance with the instruction
Failure of the device Failure to comply with instructions
Not enough oxygen for patient Usage of untrained persons and not reading manual
Encountered Problems Lack of risk assessment
Storage Concitions Failure to comply with requirements on product labels
RELATED DIRECTIVES
Medical Devices Directive 9 /42 / EEC is taken as reference.
ELECTROMAGNETIC COMPATIBILITY
Foras OXY Oxygen Concentrator complies with the electromagnetic compatibility
limitations of the medical device directive 9 /42/EEC. (En 55011 BF class and EN 60601-
1-2) IEC 60601-1-2 requirements for EMC have been completed.
15 / 18

Oxygen Concentrator User Manual
Desc iption of Manual and Manufactu e
Elect omagnetic Immunity:
The oxygen concentrator is suitable for use in the electro-magnetic environment which
described below. The user should pay attention to the environment when using the
concentrator and use it as in the declared environment.
Immunity Test IEC 60601 Test Level Compatibility Level Elect omagnetic Env.-Guide
Electrostatic Discharge
61000-4-2
± 8 kV Contact
± 15 kV Air
± 8 kV Contact
± 15 kV Air
The floor should be wood, concrete
or ceramic tiles. If the floors are
covered with synthetic material, the
humidity should be at least 0%.
Radiated RF
61000-4-
Conducted
61000-4-6
V/m
80 - 2700 Mhz
V
150 kHz - 80 Mhz
1 kHz
V
Portable and mobile RF
communications equipment should
not be used closer to any part of the
device, including cables, than the
recommended separation distance
calculated from the equation
applicable to the frequency of the
transmitter.Adviced separation
distance equation;
d = 1.2√P 150 kHz to 80 Mhz
d = 1.2√P 80 MHz to 800 Mhz
d = 2. √P 800 MHz to 2.5 Ghz
Where P is the maximum output
power rating of the transmitter in
watts (W) according to the
transmitter manufacturer and d is
the recommended separation
distance in meters (m).
The field intensity determined by
electromagnetic field research of
RF transmitters must be less than
the level of conformity in each
frequency range. Confusion may
occur with devices bearing the
following symbols on them.
Electrical rapid transient
regime / immunity against
sudden impact
61000-4-4
± 2 kV for power supply lines
± 1kV for Input Output Lines
± 2 kV for power supply
lines
± 1kV for Input Output
Lines
The power quality of the network
should be similar to that of a typical
workplace or hospital.
Pulse Immunity
61000-4-5
Lines/Line, lines / lines ±0.5 kV,
±1kV, ±2kV, Line/lines to ground
±0.5 kV, ±1kV, ±2kV
Lines/Line, lines / lines
±0.5 kV, ±1kV, ±2kV,
line/lines to ground ±0.5
kV, ±1kV, ±2kV
The power quality of the network
should be similar to that of a typical
workplace or hospital.
Mains frequency magnetic
field immunity
(50-60Hz)
61000-4-8
0 A/m 0 A/m The level of power frequency
magnetic fields should be as in
typical places, such as a normal
hospital or home environment.
Voltage dips,short
interruptions and voltage
variations immunity tests
61000-4-11
10 s(min)
0º, 45º, 90º, 1 5º, 180º, 225º,
270º and 15º
Cycle
0=0,5
0=1
70=25
0=250
The power quality of the network
should be similar to that of a typical
workplace or hospital. If the user
(Me device and Me system) needs
to continue operating despite power
grid outages (Me device and me
system), they must operate with a
power supply or battery that is not
interrupted.
Note: Higher frequency range applies at 80MHz and 800MHz.
Note: This information may not be available in all situations. Absorption caused by structures, objects and
people affects electromagnetic propagation.
16 / 18

Oxygen Concentrator User Manual
B oadcast Test Compatibility Elect omagnetic Envi onment- Guidance
RF Broadcast
CISPR 11
1. Group The device uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment
RF Broadcast
CISPR 11
Group B The device is suitable for use in all establishments, including
domestic establishments and those directly connected to the
public low-voltage network that supplies buildings used for
domestic purposes.
Harmonic Emissions
61000- -2
Group A
Voltage Change/Vibrating
Emissions
61000- -
Compliance
COMPLIANCE WITH STANDARTS
IEC 60601-1, IEC 60601-1-2, IEC 60601-1-6, IEC 60601-1-8, IEC 60601-1-11, ISO
80601-2-67, ISO 80601-2-69 standarts are referenced in the production of oxygen
concentrators.
IEC 60601-1 CLASSIFICATION
Protection type against electric shock : Class II
Protection degree against electic shock : Type BF
Degree of protection against impact and waterproof : IP 21
SOFTWARE INFORMATION
Firmware is included in class A software security class. Class A: Does not cause
injury or damage to health. The version definition is used for traceability of software
updates. Updates are performed on all models. The current version of oxygen
concentrators is 1.0.
Softwa e Diffe ence: OXY 00 model oxygen concentrator has the same visuality, same
hardware and the same physical characteristics as the OXY500 model as it goes through
the same manufacturing processes. The difference between them is software and the
OXY 00 model has the capacity to give liters of oxygen per minute, while the OXY500
model has the ability to give 5 liters of oxygen per minute.
Softwa e Ope ation: : In both models, when the power cable of the device is plugged in
and the turn on button is pressed, the device starts in 1 second. Then it starts operating
and continues to supply oxygen to the patient at the adjusted flow.
17 / 18

Oxygen Concentrator User Manual
Foras Bilgisayar Elektronik Medikal San. Tic. Ltd. Şti
Ostim O.S.B. Mahallesi 1269.Cadde No: 5 Yenimahalle – ANKARA
MADE IN TURKEY / TÜRK MALI
www.foras.com.tr / [email protected]
Tel: +90 12 95 77 66 / Fax: +90 12 95 77 67
Notified Body : Kiwa Belgelendirme Hizmetleri A.Ş.
Address: İTOSB 9. Cadde No: 15 Tepeören mevkii
Tuzla – İST. e-mail: [email protected]
Rev: 02
Doc. No: OXY0717
Rev. Date: 10.02.2020
Release Date: 10.02.2020
18 / 18
This manual suits for next models
1
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











