Genadyne Biotechnologies | DMR-06-091- Rev F
TABLE OF CONTENTS
SAFETY STANDARDS................................................................................................................4
WARNINGS ..................................................................................................................................6
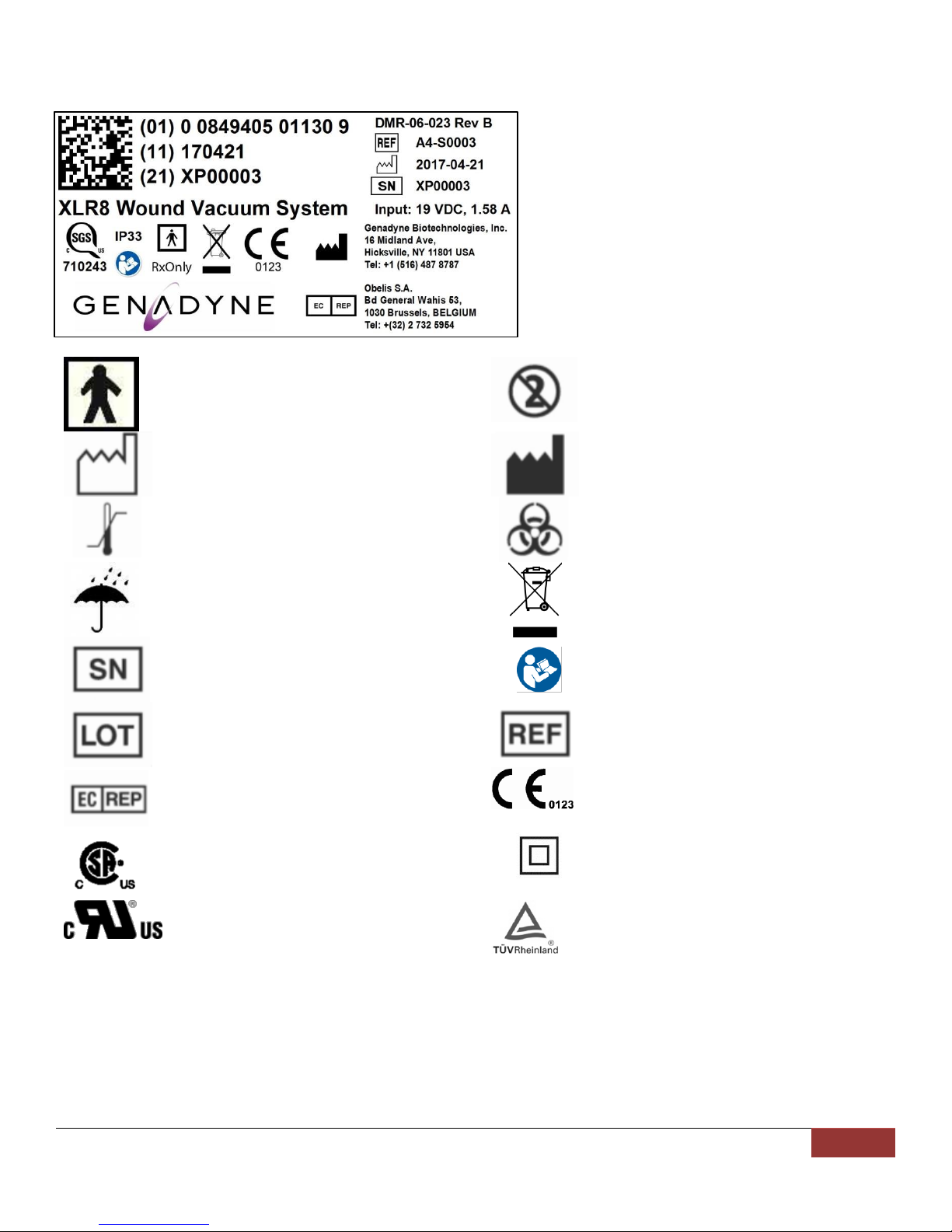
SYMBOLS.....................................................................................................................................7
INDICATION FOR USE...............................................................................................................8
USER..............................................................................................................................................8
CONTRAINDICATIONS..............................................................................................................8
PRECAUTIONS ............................................................................................................................9
STANDARD PRECAUTIONS......................................................................................................................................................................9
CONTINUOUS VERSUS VARIABLE THERAPY ...........................................................................................................................................9
PATIENT SIZE AND WEIGHT....................................................................................................................................................................9
SPINAL CORD INJURY.............................................................................................................................................................................9
BRADYCARDIA.......................................................................................................................................................................................9
ENTERIC FISTULAS.................................................................................................................................................................................9
PROTECT PERIWOUND SKIN .................................................................................................................................................................10
CIRCUMFERENTIAL DRESSING APPLICATION .......................................................................................................................................10
OPERATING PRECAUTIONS ...................................................................................................................................................................10
PHYSICIAN ORDERS................................................................................................................11
INTRODUCTION........................................................................................................................12
FEATURES..................................................................................................................................12
SYSTEM USAGE........................................................................................................................13
KEYPAD FEATURE...................................................................................................................14
OPERATING THE DEVICE.......................................................................................................15
THERAPY MODES.....................................................................................................................15
CONTINUOUS THERAPY MODE.............................................................................................................................................................15
VARIABLE THERAPY MODE .................................................................................................................................................................16
THERAPY SELECTION ...........................................................................................................................................................................17
ADJUSTING THE PRESSURE...................................................................................................................................................................18
INTENSITY MODE ....................................................................................................................18
ALERTS.......................................................................................................................................18
ENABLE /DISABLE ...............................................................................................................................................................................20
ALERT LOG ..........................................................................................................................................................................................22
ADVANCE MENU......................................................................................................................23
PREFERENCES.......................................................................................................................................................................................23
SYSTEM INFO .......................................................................................................................................................................................23
LANGUAGE SELECTION ........................................................................................................................................................................23
BATTERY POWER.....................................................................................................................24