►Report each serious incident in connection with the product, in particu
lar a worsening of the state of health, to the manufacturer and to the
relevant authority in your country.
►Please keep this document for your records.
The instructions for use provide you with important information regarding the
use of the VoltActive ankle support.
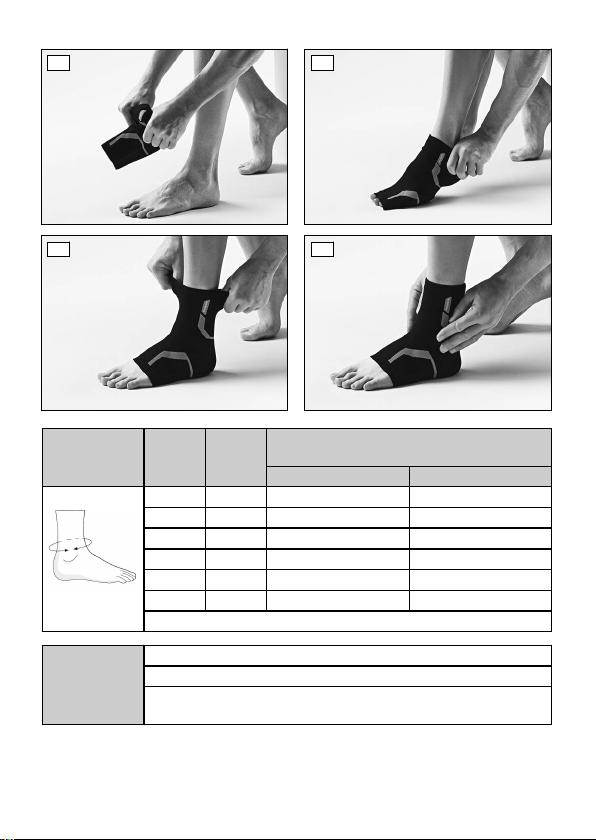
2 Intended use
2.1 Indications for use
The support is intended exclusively for orthotic fittings of the ankle and
exclusively for contact with intact skin.
The product is intended exclusively for use on one user. Use of the product
by another person is not approved by the manufacturer.
The support must be used according to the indication.
2.2 Indications
• Irritation in the ankle (postoperative, posttraumatic and chronic)
• Swelling in the ankle (postoperative, posttraumatic and chronic)
• Slight feeling of instability
• Minor ligamentous instability
• Minor ankle pain
• Fibromyalgia
• Joint effusion
• Minor to moderate ankle sprain
Indications must be determined by the physician.
2.3 Contraindications
2.3.1 Absolute Contraindications
Not known.
2.3.2 Relative Contraindications
The following indications require consultation with a physician: skin dis
eases/injuries, inflammation, prominent scars that are swollen, reddening
and hyperthermia of the fitted limb/body area; lymphatic flow disorders,
including unclear soft tissue swelling distant to the body area to which the
medical device will be applied; sensory and circulatory disorders in the foot
area.
9