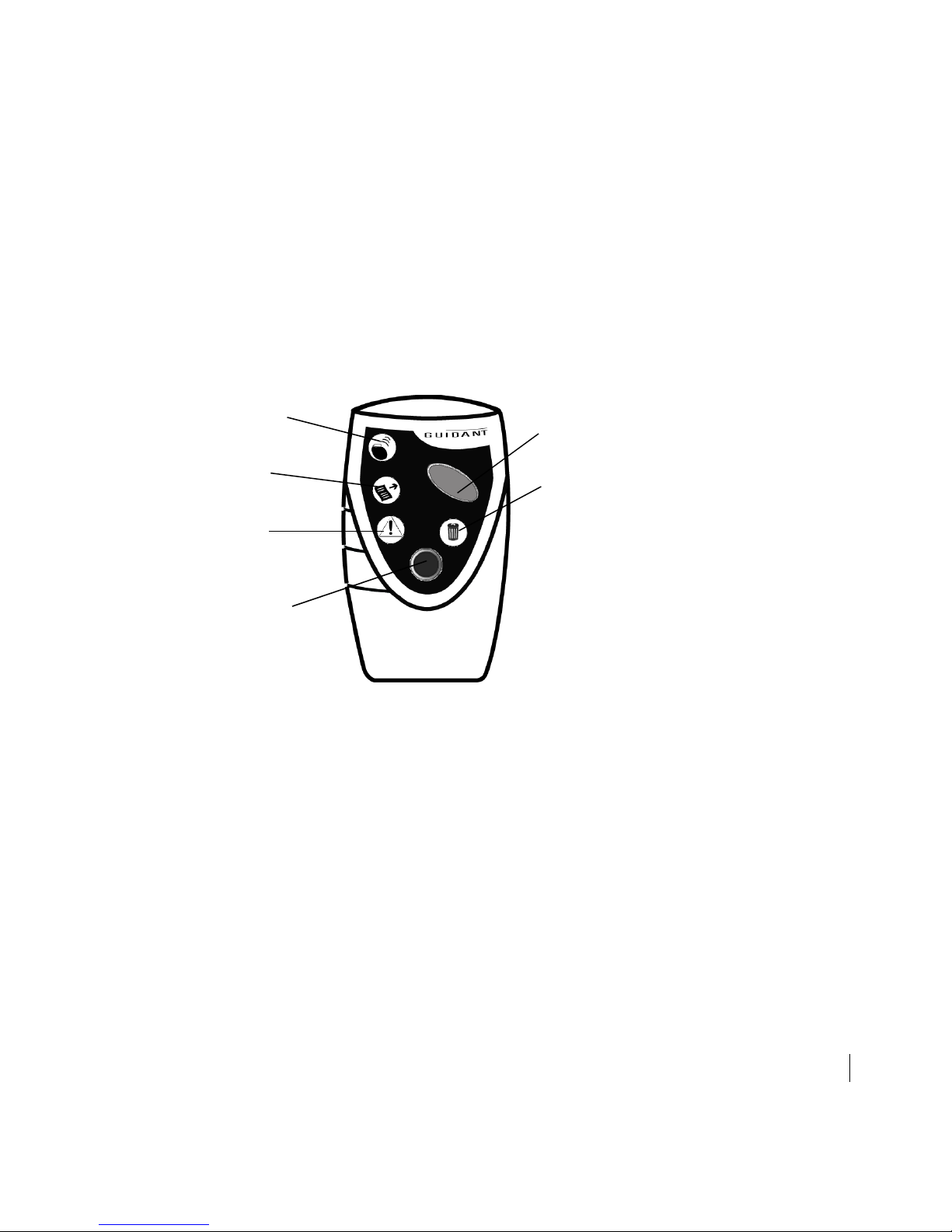
How to Interrogate an Implanted Heart Failure Device 7
will emit the “Interrogation complete” message. The Interrogation light will
stay on for five seconds after successful interrogation.
NOTES:
• You have about 10 seconds from the time you press a button until you locate the
implanted device. If the HF PARTNER device is unable to locate the device, the
voice command “Locate implanted device” will be repeated. Position the top half
of the HF PARTNER device over the implanted device and move the
HF PARTNER device slowly. If the interrogation is unsuccessful, hold the
HF PARTNER device over the patient's implanted devices with the button and
lights AWAY from you.
• If the interrogation was unsuccessful, the HF PARTNER device will notify you by
communicating “Interrogation unsuccessful, please interrogate again”. Repeat
the interrogation process until you receive the “Interrogation complete” message.
• If you hear the “Locate implanted device” message (NOT followed by the
“Interrogation unsuccessful, please interrogate again” message), you have
requested the interrogation of an incompatible device. Ensure that you have a
compatible Guidant device.
• If communication is lost during interrogation, the visual indicators on the front of
the HF PARTNER device will light in consecutive sequence. Reposition the
Figure 3. Hold the HF PARTNER device over the patient’s implanted device, with
buttons and lights toward you.