Fingertip
Pulse Oximete
USER MANUAL
General Description
Oxygen binds to hemoglobin in red blood cells when moving through the lungs. It is transported throughout the body as arterial
blood. A pulse oximeter uses two frequencies of light (red and infrared) to determine the percentage (%) of hemoglobin in the blood
that is saturated with oxygen. The percentage is called blood oxygen saturation, or SpO2. A pulse oximeter also measures and
displays the pulse rate at the same time it measures the SpO2level.
Measurement Principle
Principle of the oximeter is as follows: The pulse oximeter works by applying a sensor to a pulsating arteriolar vascular bed. The
sensor contains a dual light source and photo detector. The one wavelength of light source is 660nm, which is red light; the other is
905nm, which is infrared-red light. Skin, bone, tissue and venous vessels normally absorb a constant amount of light over time. The
photo detector in finger sensor collects and converts the light into electronic signal which is proportional to the light intensity. The
arteriolar bed normally pulsates and absorbs variable amounts of light during systole and diastole, as blood volume increases and
decreases. The ratio of light absorbed at systole and diastole is translated into an oxygen saturation measurement. This
measurement is referred to as SpO2.
Diagram of Operation Principle
1. Red and Infrared-ray Emission Tube
2. Red and Infrared-ray Receipt Tube
Precautions For Use
1. Before use, carefully read the manual.
2. Operation of the fingertip pulse oximeter may be affected by the use of an electrosurgical unit (ESU).
3. The fingertip pulse oximeter must be able to measure the pulse properly to obtain an accurate SpO2measurement. Verify
that nothing is hindering the pulse measurement before relying on the SpO2measurement.
4. Do not use the fingertip pulse oximeter in an MRI or CT environment.
5. Do not use the fingertip pulse oximeter in situations where alarms are required. The device has no alarms. It is not for
continuous monitoring.
6. Do not use the fingertip pulse oximeter in an explosive atmosphere.
7. The fingertip pulse oximeter is intended only as an adjunct in User assessment. It must be used in conjunction with other
methods of assessing clinical signs and symptoms.
8. In order to ensure correct sensor alignment and skin integrity, the maximum application time at a single site for our device
should be less than half an hour.
9. Do not sterilize the device using autoclaving, ethylene oxide sterilizing, or immersing the device in liquid. The device is not
intended for sterilization.
10. Follow local ordinances and recycling instructions regarding disposal or recycling of the device and device components,
including batteries.
11. This equipment complies with IEC 60601-1-2:2007 for electromagnetic compatibility for medical electrical equipment and/or
systems. However, because of the proliferation of radio-frequency transmitting equipment and other sources of electrical
noise in healthcare and other environments, it is possible that high levels of such interference due to close proximity or
strength of a source might disrupt the performance of this device.
12. Portable and mobile RF communications equipment can affect Oximeter electrical equipment.
13. This equipment should not be used adjacent to or stacked with other equipment.
14. It may be unsafe to:
—use accessories、detachable parts and materials not described in the instructions for use
—interconnect this equipment with other equipment not described in the instructions for use
—disassemble, repair or modify the equipment.
15. These materials that contact with the User’s skin contain medical silicone and ABS plastic enclosure are all pass the
ISO10993-5 Tests for in vitro cytotoxicity and ISO10993-10 Tests for irritation and delayed-type hypersensitivity.
Contraindication
It is not for continuous monitoring.
Inaccurate measurements may be caused by
1.Significant levels of dysfunctional hemoglobin (such as carbonyl - hemoglobin or methemoglobin).
2.Intravascular dyes such as indocyanine green or methylene blue.
3.High ambient light. Shield the sensor area if necessary.
4.Excessive user movement.
5.High-frequency electrosurgical interference and defibrillators.
6.Venous pulsations.
7.Placement of a sensor on an extremity with a blood pressure cuff, arterial catheter, or intravascular line.
8.The user has hypotension, severe vasoconstriction, severe anemia, or hypothermia.
9.The user is in cardiac arrest or is in shock.
10.Fingernail polish or false fingernails.
11.Weak pulse quality (low perfusion).
12.Low hemoglobin.
Product Features
1 High brightness LED/LCD display SpO2, PR, and Pulse bar.
2 Two display modes.
3 2 pcs AAA-size alkaline batteries; battery-low indicator.
4 When no operation or low signal is detected, the pulse oximeter will power off automatically in 8 seconds.
Intended Use
Fingertip pulse oximeter is a portable non-invasive device intended for spot-checking of oxygen saturation of arterial hemoglobin
(SpO2) and pulse rate of adult and child in sports and/or aviation use. It is not for continuous monitoring or medical use.
Operation Instructions
1.Install two AAA batteries according to the Battery Installation instructions.
2.Place one of your fingers into the rubber opening of the pulse oximeter.
3.Press the switch button one time on front panel to turn the pulse oximeter on.
4.Keep your hands still for the reading. Do not shake your finger during the test. It is recommende
that you do not move your body while taking a reading.
5.Read the data from the display screen. There are two display modes. After turning on the pulse oximeter, each time you
press the power switch, the pulse oximeter will switch to another display modes.
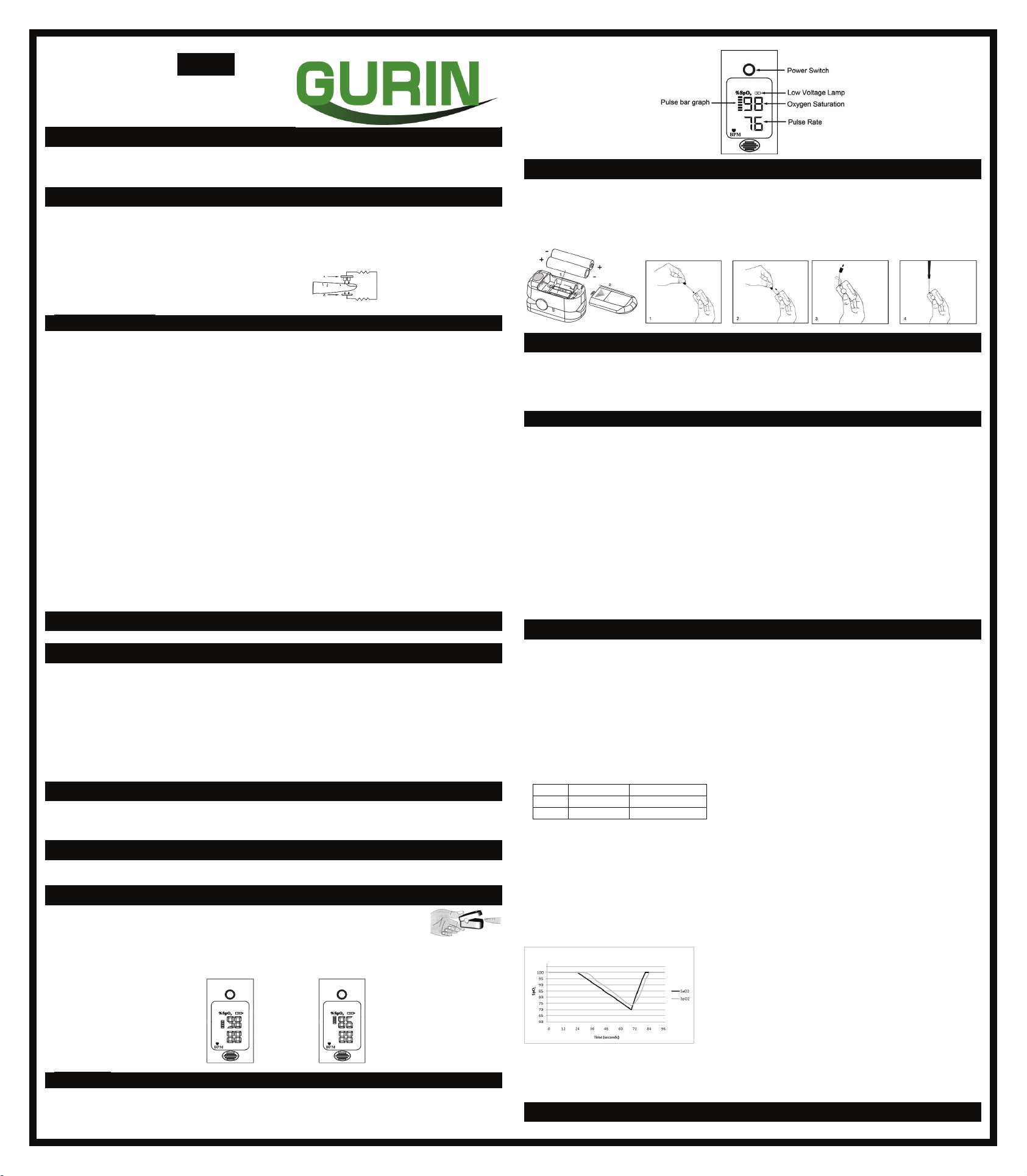
Front Panel
The pulse bar less than 30% indicates signal inadequacy and the displayed SpO2and pulse rate value is potentially incorrect.
Battery Installation
1. Install two AAA batteries into the battery compartment. Match the plus (+) and minus (-) signs in the compartment. If the
polarities are not matched, damage may be caused to the oximeter.
2. Slide the battery door cover horizontally along the arrow shown as the picture.
Note:
Please remove the batteries if the pulse oximeter will not be used for long periods of time.
Please replace the battery when the power indicator starting flickering.
Using the Lanyard
1. Thread thinner end of the lanyard through the loop.
2. Thread thicker end of the lanyard through the threaded end before pulling it tightly.
Warnings!
Keep the oximeter away from young children. Small items such as the battery door, battery, and lanyard are choking hazards.
Do not hang the lanyard from the device’s electrical wire.
Please notice that the lanyard which is tied to the oximeter may cause strangulation due to excessive length.
Maintenance and Storage
1. Replace the batteries in a timely manner when low voltage lamp is lighted.
2. Clean surface of the fingertip oximeter before it is used in diagnosis for user.
3. Remove the batteries if the oximeter is not operated for a long time.
4. It is best to store the product in -25℃~+70℃and ≤93% humidity.
5. Keep in a dry place. Extreme moisture may affect oximeter lifetime and may cause damage.
6. Dispose of battery properly; follow any applicable local battery disposal laws.
Cleaning the fingertip pulse oximeter
Please use medical alcohol to clean the silicone touching the finger inside of oximeter with a soft cloth dampened with 70%
isopropyl alcohol. Also clean the being tested finger using alcohol before and after each test.
Do not pour or spray liquids onto the oximeter, and do not allow any liquid to enter any openings in the device. Allow the oximeter to
dry thoroughly before reuse.
The fingertip pulse oximeter requires no routine calibration or maintenance other than replacement of batteries.
The use life of the device is five years when it is used for 15 measurements every day and 10 minutes per one
measurement. Stop using and contact local service center if one of the following cases occurs:
An error in the Possible Problems and solutions is displayed on screen.
The oximeter cannot be powered on in any case and not the reasons of battery.
There is a crack on the oximeter or damage on the display resulting readings cannot be identified; the spring is invalid; or the
key is unresponsive or unavailable.
Specifications
1. Display Type
LED display
2. SpO2
Display range: 0%~100%
Measurement range: 70%~100%
Accuracy: 70%~100%±2digits; 0%~69% no definition
Resolution: 1%
3. Pulse Rate
Display range: 0bpm~250bpm
Measure range: 30bpm~250bpm
Accuracy: 30bpm~99bpm, ±2bpm; 100bpm~250bpm, ±2%
Resolution: 1bpm
4. Probe LED Specifications
Wavelength Radiant Power
RED 660±3nm 3.2mW
IR 905±10nm 2.4mW
NOTE: The information about wavelength range can be especially useful to clinicians.
5. Power Requirements
Two AAA alkaline Batteries
Power consumption: Less than 40mA
Battery Life: Two AAA 1.5V, 1200mAh alkaline batteries could be continuously operated as long as 18 hours.
6. Environment Requirements
Operation Temperature: 5℃~40℃
Storage Temperature: -25℃~+70℃
Ambient Humidity: 15%~93% no condensation in operation; ≤93% no condensation in storage/transport
Atmosphere pressure: 70kPa~106kPa
7. Equipment data update period
As shown in the following figure. Data update period of slower average is 8s.
8. Classification
According to the type of protection against electric shock: INTERNALLY POWERED EQUIPMENT;
According to the degree of protection against electric shock: TYPE BF APPLIED PART, (applied part: the rubber hole of the device);
According to the degree of protection against ingress of water: IPX22
According to the mode of operation: CONTINUOUS OPERATION
Clinical Study Summary
M11

