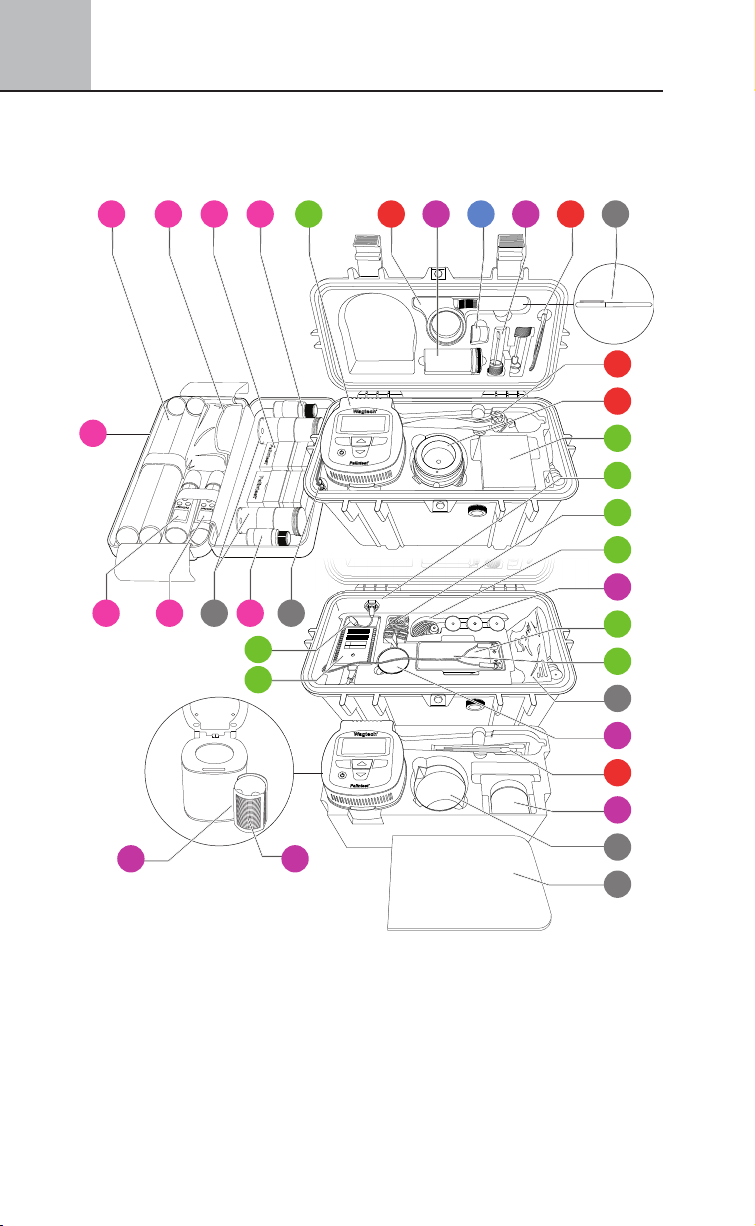
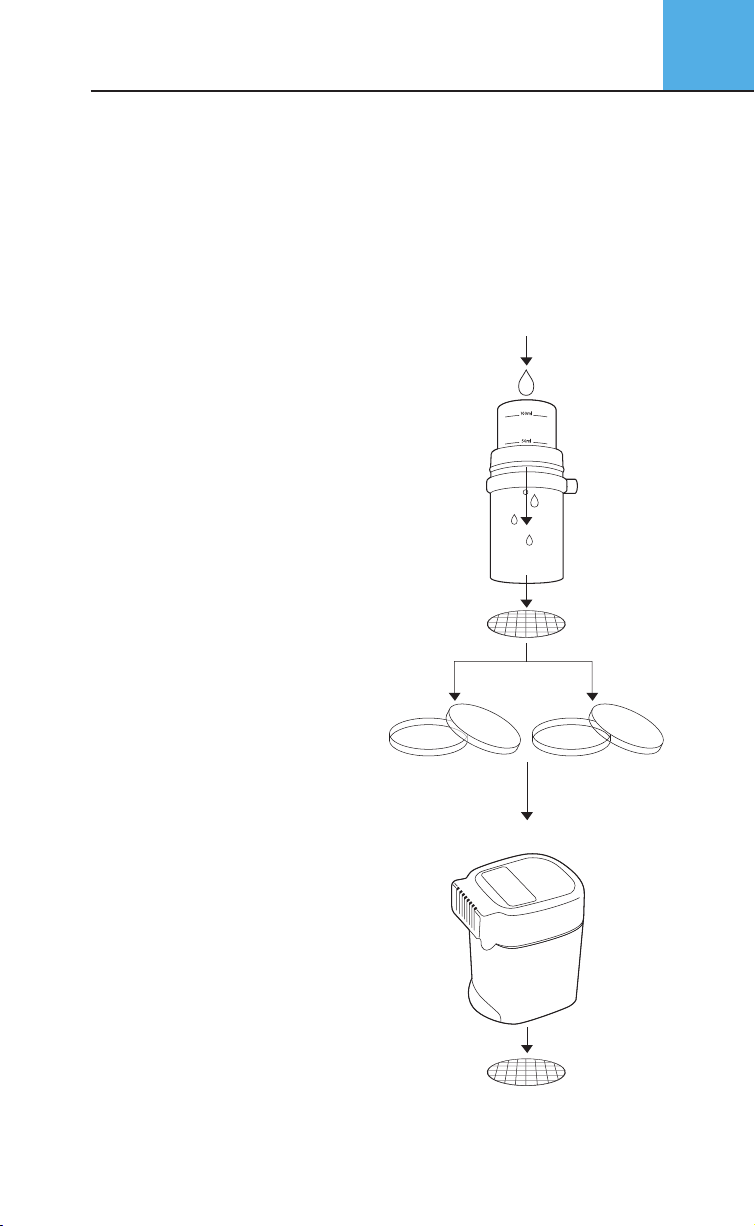
6
2Introduction
2.0 Introduction
The Wagtech PotatestTM is a portable water quality test kit. It has been designed primarily to
test the microbiological quality of drinking water; assessing whether or not there has
been faecal contamination of a water source. It allows the end user to test directly for Total
or aecal Coliforms as well as the critical indicators of microbiological quality: Turbidity,
pH and ree and Total Chlorine.
The Wagtech PotatestTM conforms to advice given by the World Health Organisation
(WHO) for the field based testing of microbiological water quality. The parameters measured
for, and techniques/procedures used are based on accepted laboratory methods and are
adapted for use in demanding field conditions.
More information on the “WHO uidelines for Drinking Water Quality” can be
found at www.palintest.com
As with all the kits in the Wagtech range, ease of use is integral to the design. The Wagtech
PotatestTMis suitable for use by technicians of all skill levels and this manual provides the
essential information required to conduct rapid water quality testing in the field.
This instruction manual is also available in rench, Spanish and Mandarin.
Additional advice and training is available upon request. Contact us directly at
2.1 Before You Use Your Kit
2.1.1 Microbiological
Analysis of Drinking Water
Drinking water contaminated by faecal
matter may contain pathogenic (disease
causing) organisms and represent a risk
to public health.
It is impractical to attempt to isolate specific
pathogens because they are present in
relatively small numbers compared with
other types of microorganisms. Moreover,
there are many types of pathogen and each
requires a unique microbiological isolation
technique. The accepted approach is to
analyse for indicator organisms that inhabit
the gut in large numbers and are excreted in
human/animal faeces. The presence of these
indicator organisms in water is evidence of
faecal contamination and therefore a risk
that pathogens are present. If indicator
organisms are present in large numbers,
the contamination is considered to be
recent and/or severe.
The group of indicator bacteria tested for with
the PotatestTM are called Coliforms; more
specifically the focus is on the enumeration of
Thermotolerant Coliforms (sometimes called
aecal Coliforms). These are bacteria that
originate from faecal sources. However the
PotatestTM is also capable of testing for
Total Coliforms by simply selecting a different
incubation temperature.
Thermotolerant Coliforms or aecal Coliforms
are used in water microbiological testing
to denote coliform organisms which grow
at 44 or 44.5°C and ferment lactose to
produce acid and gas.
In practice, some organisms with these
characteristics may not be of faecal origin
and the term Thermotolerant Coliforms is
therefore more correct and is becoming
more commonly used. Nevertheless the
presence of Thermotolerant Coliforms nearly
always indicates faecal contamination.
Usually, more than 95% of Thermotolerant
Coliforms isolated from water are the gut
organism Escherichia coli (E. coli), the
presence of which is definitive proof
of faecal contamination.
As a result, it is often unnecessary to
undertake further testing to confirm the
specific presence of E. coli.