Hemochron Response User manual

Operator’s Manual
English
TABLE OF CONTENTS
INTENDED USE.............................................................. 2
SUMMARY AND EXPLANATION.................................... 2
PRINCIPLES OF OPERATION ....................................... 2
ATTENTION LABEL ........................................................ 4
SPECIFICATIONS........................................................... 5
GETTING STARTED....................................................... 5
SETTING SUPERVISOR OPTIONS ............................. 11
SETTING OUTPUT OPTIONS ...................................... 18
SETTING PROGRAM OPTIONS .................................. 20
CUSTOMIZING THE PRINTED HEADING ................... 21
OPERATION ................................................................. 21
QUALITY CONTROL (QC)............................................ 27
OPERATING PRECAUTIONS....................................... 29
LIMITATIONS ................................................................ 29
RESULTS MANAGEMENT ........................................... 30
DEFAULT SETTINGS ................................................... 32
TROUBLESHOOTING .................................................. 33
SYSTEM TESTS ........................................................... 35
MAINTENANCE ............................................................ 38
SPECIFICATIONS FOR PERIPHERALS ...................... 39
SAFETY STANDARDS ................................................. 41
INDEX ........................................................................... 42
This manual is published by International Technidyne Corporation (ITC) for use with the HEMOCHRON
Response V2.00 or above. Questions or comments regarding the contents of this manual can be directed to
the address at the back of this manual or to your ITC representative.
HEMOCHRON
®
and RxDx
®
are registered trademarks of ITC.
idms™ is a trademark of ITC.
Celite
®
is registered trademark of Celite Corporation.
©2000, 2001, 2002, 2003, 2004. This document is the copyright of ITC and must not be copied or
reproduced in any form without prior consent. ITC reserves the right to make technical improvements to
this equipment and documentation without prior notice as part of a continuous program of product
development.
Printed From ITC Intranet

2
INTENDED USE
The HEMOCHRON
®
Response Whole Blood Coagulation System is a dual-well microprocessor-controlled
coagulation testing instrument with an integral test type barcode reader, RS232 communication interface
capability, and a printer. The system runs coagulation tests such as Activated Clotting Time (ACT), Activated
Partial Thromboplastin Time (APTT), Prothrombin Time (PT) and other specialty tests that are currently
available from ITC.
SUMMARY AND EXPLANATION
Events that lead to formation of a blood clot are simplified in coagulation theory into two interactive
coagulation cascades. The Activated Clotting Time (ACT), Activated Partial Thromboplastin Time (APTT) and
Prothrombin Time (PT) tests are general coagulation screening tests that are used to measure the
functionality of these cascades.
The ACT test is the method of choice for monitoring heparin therapy. Administration of heparin to maintain
hemostasis during cardiac surgery and cardiac angioplasty procedures can pose significant risk to the
patient. Since individual patients can vary as much as twelve-fold in heparin sensitivity, overdosing heparin
can result in dangerous bleeding and underdosing heparin can lead to thrombosis.
ACT is performed by adding a clotting activator such as Celite
®
, silica, kaolin, or glass particles to a blood
sample and then measuring the length of time required for clot formation. The particular clotting activator
that is used influences the time required for clot formation. Celite (diatomaceous earth) is the standard ACT
reagent used for high level heparin monitoring because of its excellent activating properties. However,
serine protease inhibitors such as aprotinin that may be administered to certain patients to decrease
postoperative bleeding can prolong the Celite activated ACT. When aprotinin is on-board, a kaolin-activated
ACT tube should be used.
The APTT test measures the intrinsic coagulation pathway and involves all coagulation factors except factors
VII and III (tissue factor). The APTT test improves the earlier PTT test through use of a contact activating
substance which standardizes activation of Factor XII to provide a more precise and sensitive assay for low
level heparin monitoring.
The PT test measures the extrinsic coagulation pathway and is sensitive to coagulation factors VII, X, V, II,
and fibrinogen. PT results may be abnormal in patients with liver disease or Vitamin K deficiency, and the
test is widely used to monitor oral anticoagulant therapy.
Under clinical conditions, the coagulation cascade may be affected by either naturally occurring or
administered procoagulants or anticoagulants. Endogenous changes in hemostasis, such as disseminated
intravascular coagulation, can result in extreme clotting factor depletion. In order to determine which
pathway is being affected, a panel of coagulation assays may be performed. Results of these tests are used to
diagnose the hemostatic abnormality and to determine the appropriate therapeutic intervention.
PRINCIPLES OF OPERATION
The patented HEMOCHRON clot detection module contains two test wells into which disposable unitized
coagulation test tubes can be inserted. The test tubes (provided in a separately purchased test kit) contain
reagents for a particular test and a precision magnet. Immediately after the sample is added to the test tube,
the START button is pressed, the test tube is agitated, and the test tube is placed into the test well by the
operator. There, it is automatically rotated at a controlled speed and incubated at 37 °C ±1.0 °C.
When a fibrin clot begins to form, it causes the magnet in the test tube to be displaced. Two magnetic
detectors located in the test well continuously monitor the precise magnet position. When a specific
displacement of the magnet occurs, the elapsed time between the beginning of the test and the clot
endpoint is displayed as the coagulation time (in seconds). The instrument also emits an audible beep
when clot formation occurs, indicating the end of the test.
The coagulation time is displayed on the LCD screen. The operator may choose to print the result (if
automatic printing of results is not specified) or simply proceed to the next desired assay.
Printed From ITC Intranet
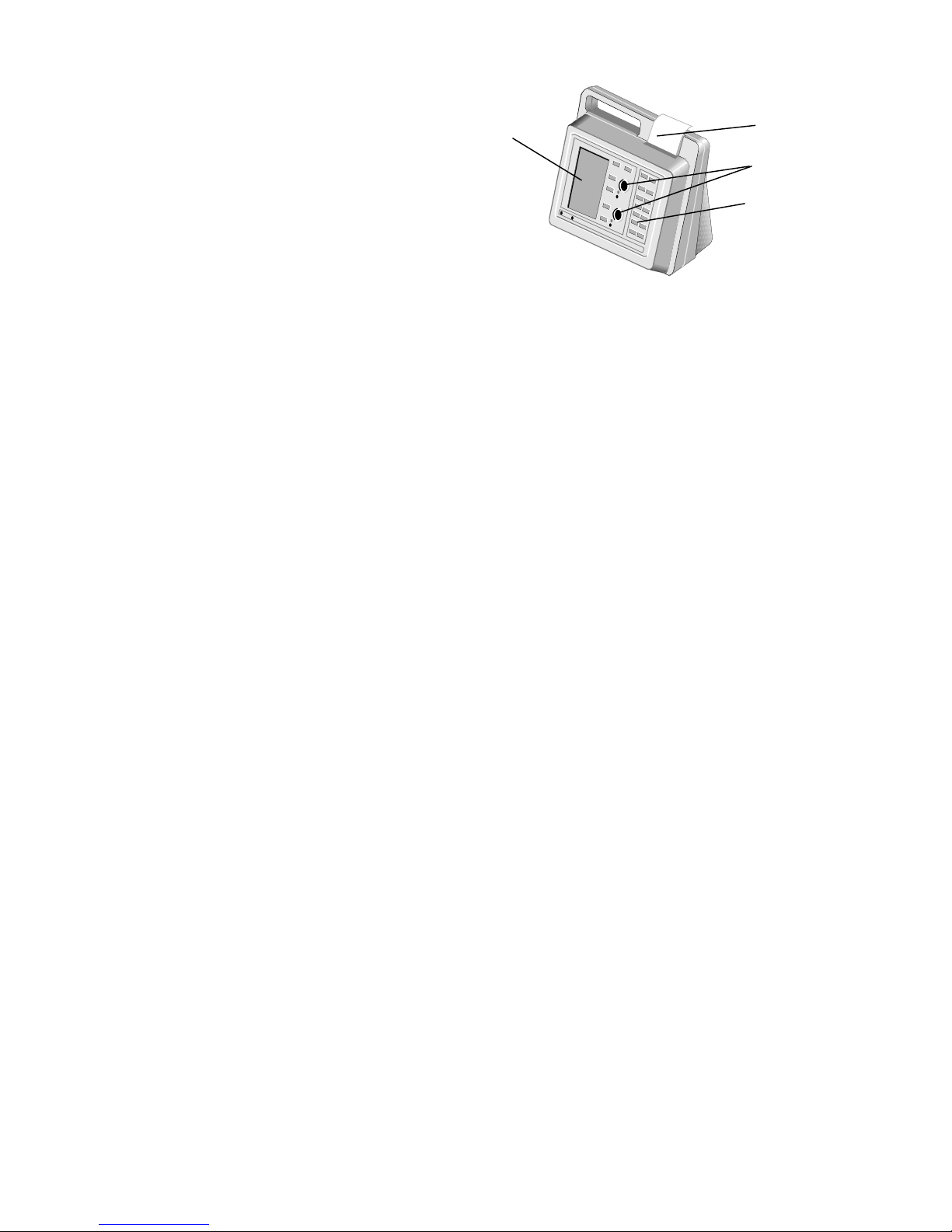
3
The system (Figure 1) contains a dual-well patented clot detection module. User interface is through a
keypad and display panel. Test results are shown on the display panel on completion of the test, and can be
printed.
Figure 1. HEMOCHRON Response Whole Blood Coagulation System
Two RS232 serial ports and a Centronix parallel port are included so that results and other information
from the data storage module can be downloaded to the laboratory computer or printed elsewhere. The
RS232 serial ports can also be used to connect an external barcode reader for importing Patient ID (PID)
and/or Operator ID (OID).
Definitions and Terms
The following acronyms and abbreviations are used in this manual, instrument screens and printouts:
ACT Activated Clotting Time
APTT Activated Partial Thromboplastin Time
DB Database
ESV Electronic System Verification
HRDM HEMOCHRON Response Data Manager software program
idms Integrated Data Management System
INR International Normalized Ratio
LQC Liquid Quality Control
OID Operator Identification Number
PIN Operator Personal Identification Number
PID Patient Identification Number
POCC Point of Care Coordinator
PPID Fibrinogen Product Performance ID Code
PT Prothrombin Time
QC Quality Control
TVT Temperature Verification Tube
Overview of Operation
Important: Disposable ready-to-use test tubes for use with the system can be obtained from ITC. Test
tubes from other manufacturers can be run, but the test being run will not be identified and the
clotting time results may be significantly different. The laboratory should verify performance if test
tubes from other manufacturers are used.
The system rotates the test tube at a constant rate while continuously monitoring the contents. An
integrated test type barcode reader decodes the test name and expiration date imprinted on the tube label.
Printer
Test
Wells
Keypad
Display
Panel
Printed From ITC Intranet

4
After a clot forms, the instrument beeps and the clotting time is displayed on the display panel. The result is
also stored in the system database with the date and time the test was performed, and the assay type. If
entered, the PID and OID are stored with the test result.
Features
The system has a number of performance and convenience features:
• The system is portable for bedside use
• A multi-test menu is resident on the system
• Fresh whole blood or citrated whole blood can be used with the appropriate test tubes
• A sample size of up to 2 mL of whole blood is required
• Test name and expiration date are automatically read when ITC barcoded test tubes are used
• Successful or errant results are automatically stamped with date and time
• Results are available in minutes
• Results are displayed appropriately as whole blood or plasma equivalent or INR (PT assay only)
• Results of 600 patient tests and 300 QC tests can be stored for each well, with optional entry of
PID, OID, and user notes
• Dose-response calculations are performed with the RxDx
®
module (if activated)
• 504 operator identification codes can be stored with OID/PIN and permissions
• Operator lockout can be configured by OID, valid OID, or PIN, using HRDM V3.0 or higher
software or the keypad
• QC lockout can be configured at one or two levels by time interval
• Stored results can be reviewed by test type, PID, OID, or date
• Stored results can be downloaded to a personal computer
• System self checks are automatically performed
• An ESV tube is available to check test well operation and detector electronics
• A Temperature Verification Tube (TVT) can be additionally used to check test well temperature
• The display is illuminated for viewing in low light
• The display can indicate the percentage of battery power remaining numerically or graphically
• The user is alerted when the battery is low
• The system includes an on-board printer
• Two external serial ports and a Centronics parallel port are provided
• Patient/QC test reports can be created using a personal computer and ITC data management
software programs
ATTENTION LABEL
An attention label on the rear of the HEMOCHRON Response instrument alerts users to accompanying
documentation:
Before using the HEMOCHRON Response instrument, it is essential that the contents of this Operator’s
Manual are read and understood by the operator.
Handle and open the container with care.
Printed From ITC Intranet

5
SPECIFICATIONS
Specifications for the HEMOCHRON Response Whole Blood Coagulation System are listed below.
Dimensions and Weight
Depth 19 cm (7.5 in)
Width 27 cm (10.5 in)
Height 22 cm (8.7 in)
Weight 2.90 kg (6.4 lbs)
Operation
Test Wells 2
Timing Range 22 seconds to 1500 seconds
Incubation Temperature 37 °C ±1.0 °C
Incubation Warm-Up Time 30 seconds to 90 seconds
Full-Charge Operating Time 8 hours (minimum)
Battery Life 500 recharges
Throughput (Full Charge) 49 test cycles (at 150 sec per test)
17 test cycles (> 500 sec per test)
AC/DC Power Module
Input Power 90 to 264 VAC, 50/60 Hz, 1.2 Amps maximum
Output Power +12 Volts DC, 3.5 Amps maximum (42 Watts,
144 BTU/hr)
Environmental
Ambient Temperature 15 to 30 °C
Note: For more technical information, refer to the HEMOCHRON Response Whole Blood Coagulation
System Service Manual.
GETTING STARTED
Unpacking and Inspection
Before unpacking the system, determine the area where the system will be located. You will need a level and
flat area that is approximately 30 cm (12 in) wide, 30 cm (12 in) deep, and 30 cm (12 in) high.
To Unpack the Instrument:
1.
Unpack the carton.
2.
Inspect each component for damage when unpacking. If damage is observed, contact your
shipper or service representative immediately.
3.
Place the instrument where it is to be located.
4.
Remove protective packaging.
5.
Examine the packaging material to be sure that the power supply, connecting cables or other
components have been removed. The materials that are provided are listed on the following page.
Note: Do not discard the packing material. It should be kept for shipping the instrument to
ITC, if repair is necessary.
Printed From ITC Intranet
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











