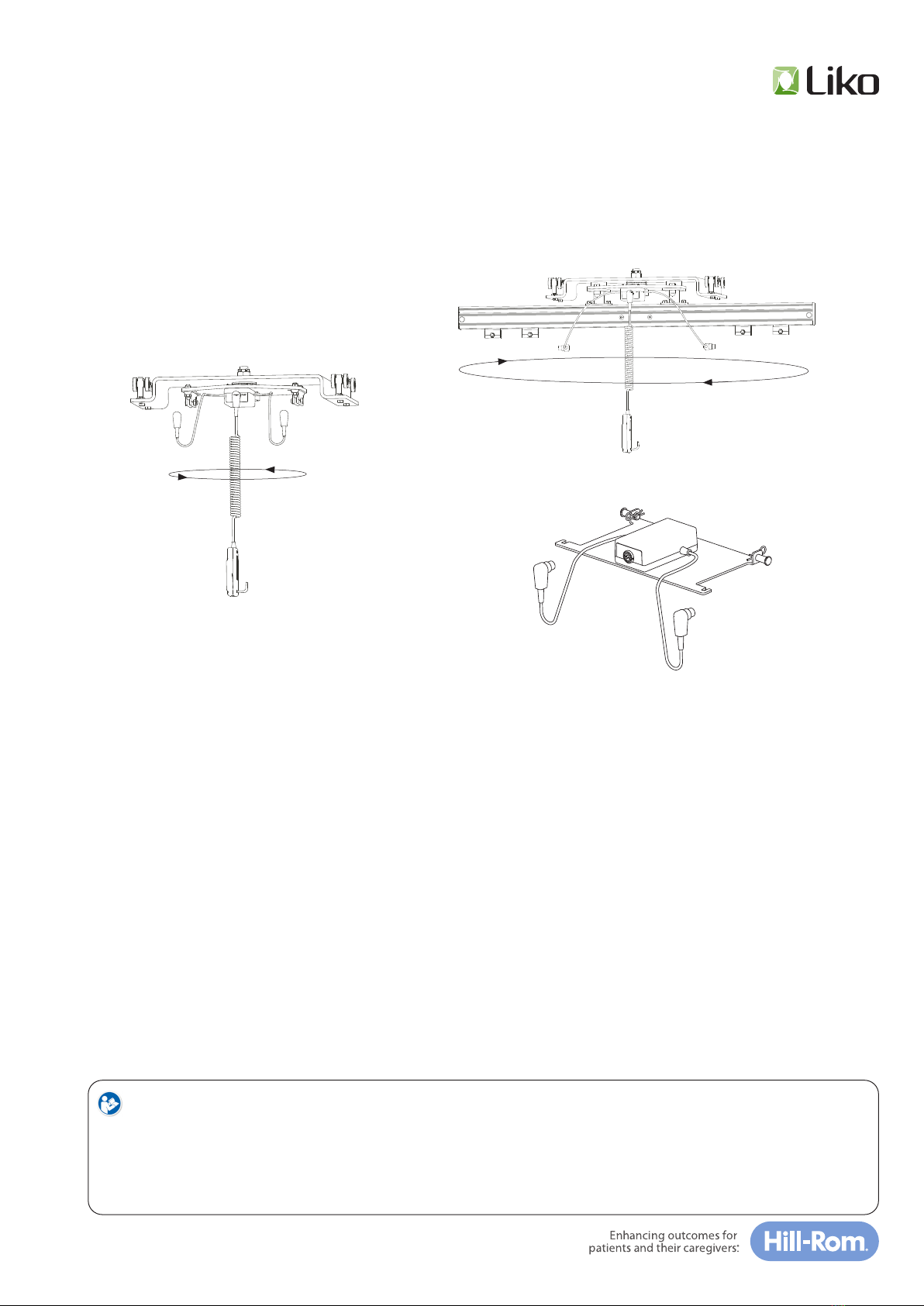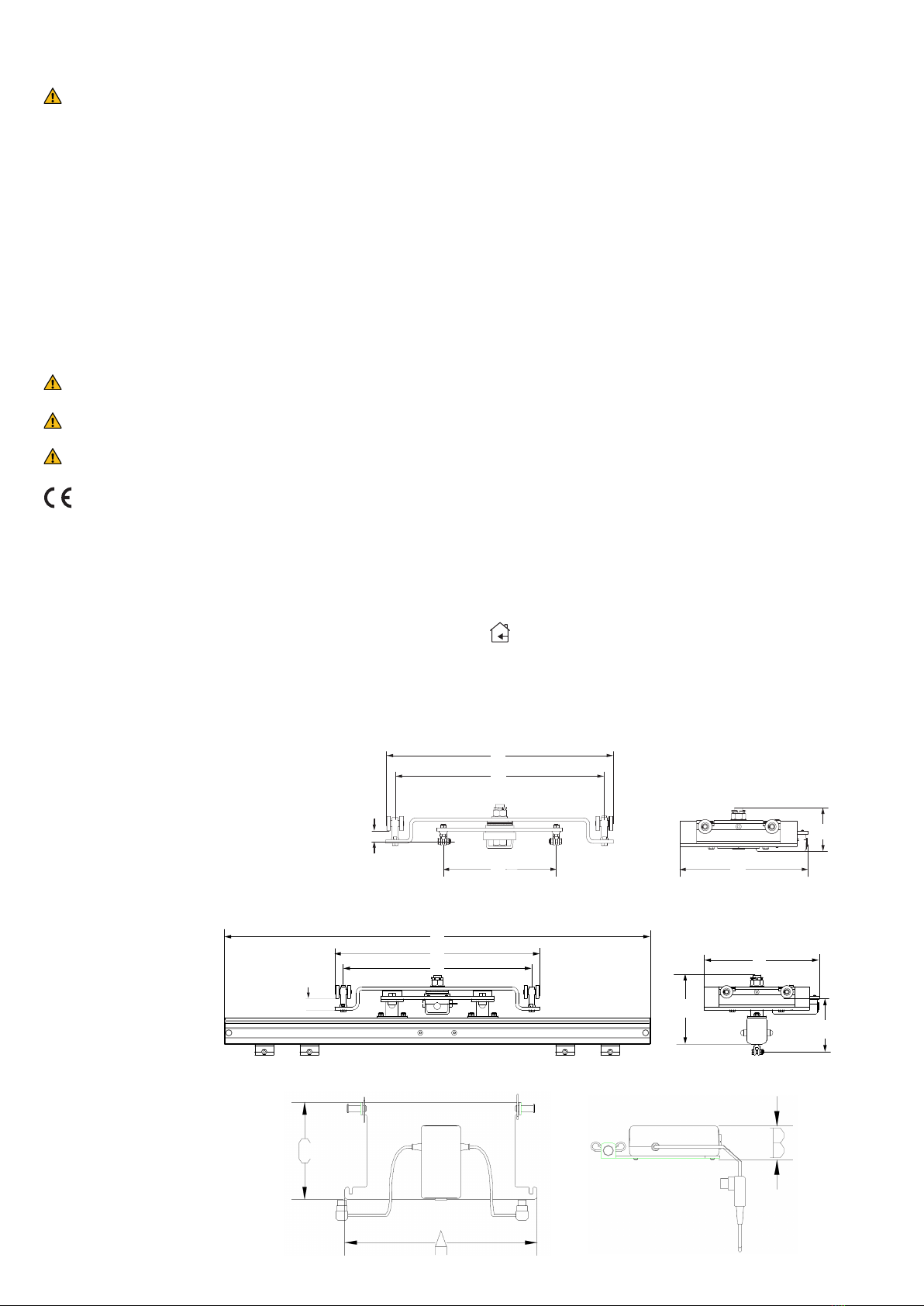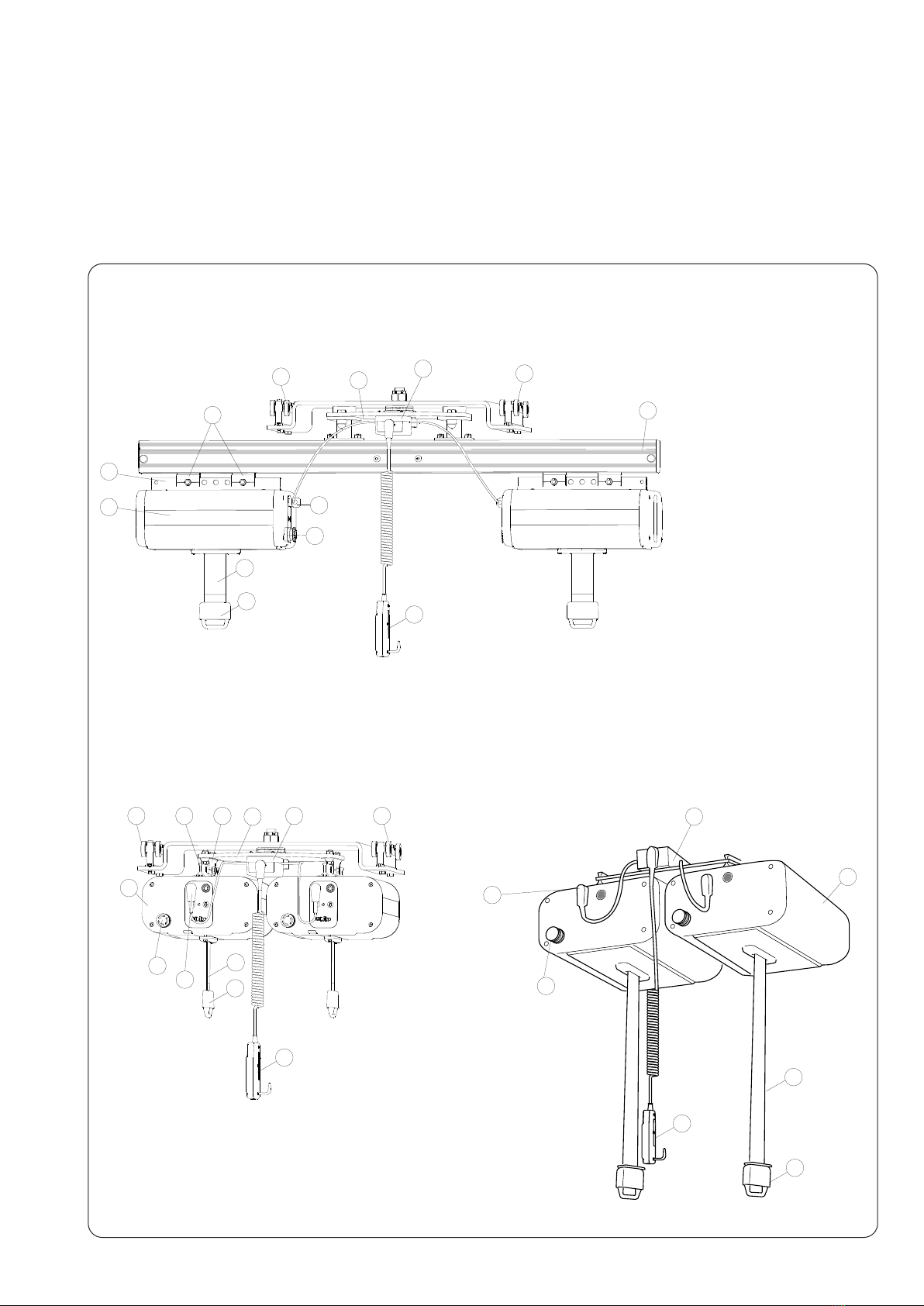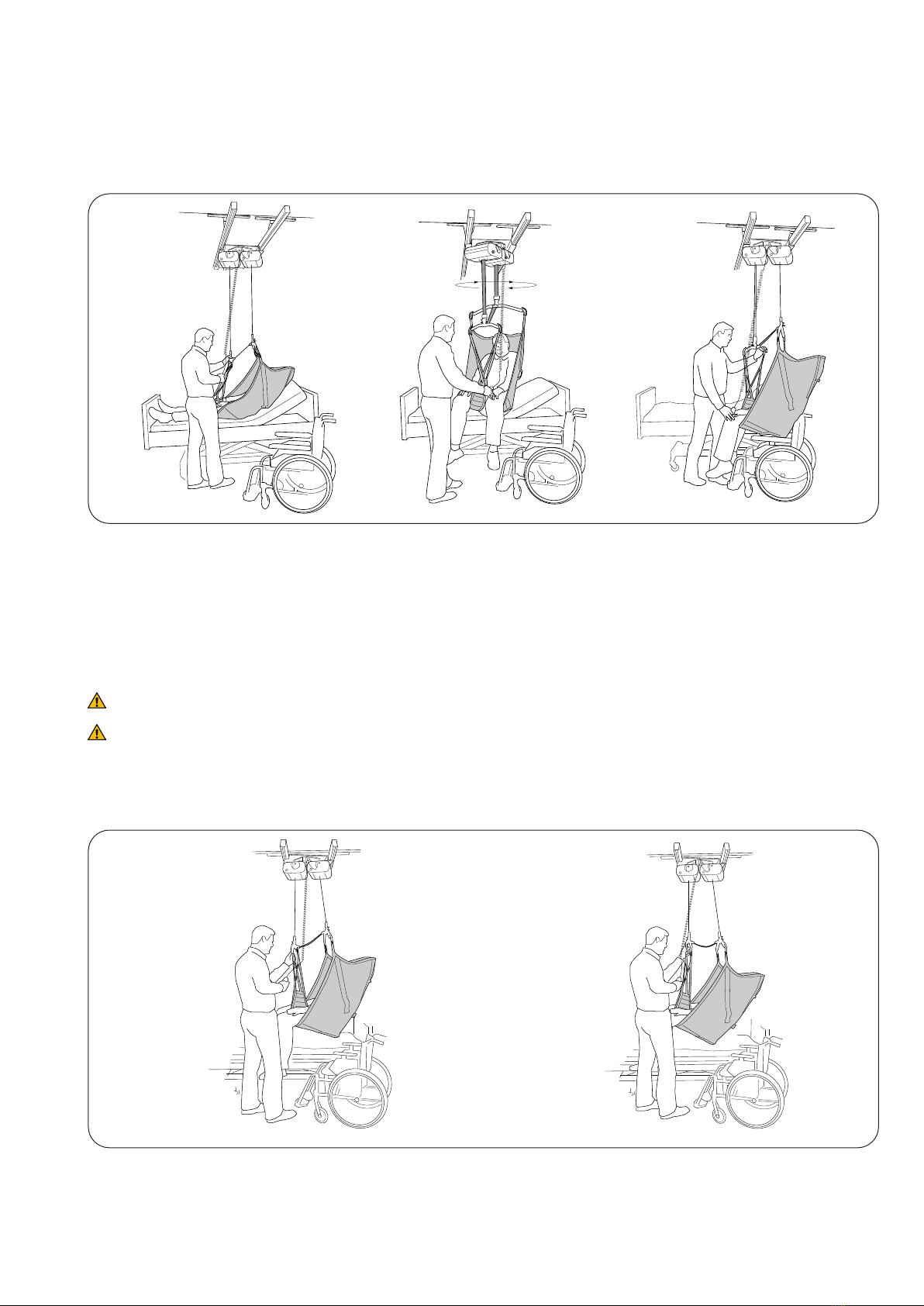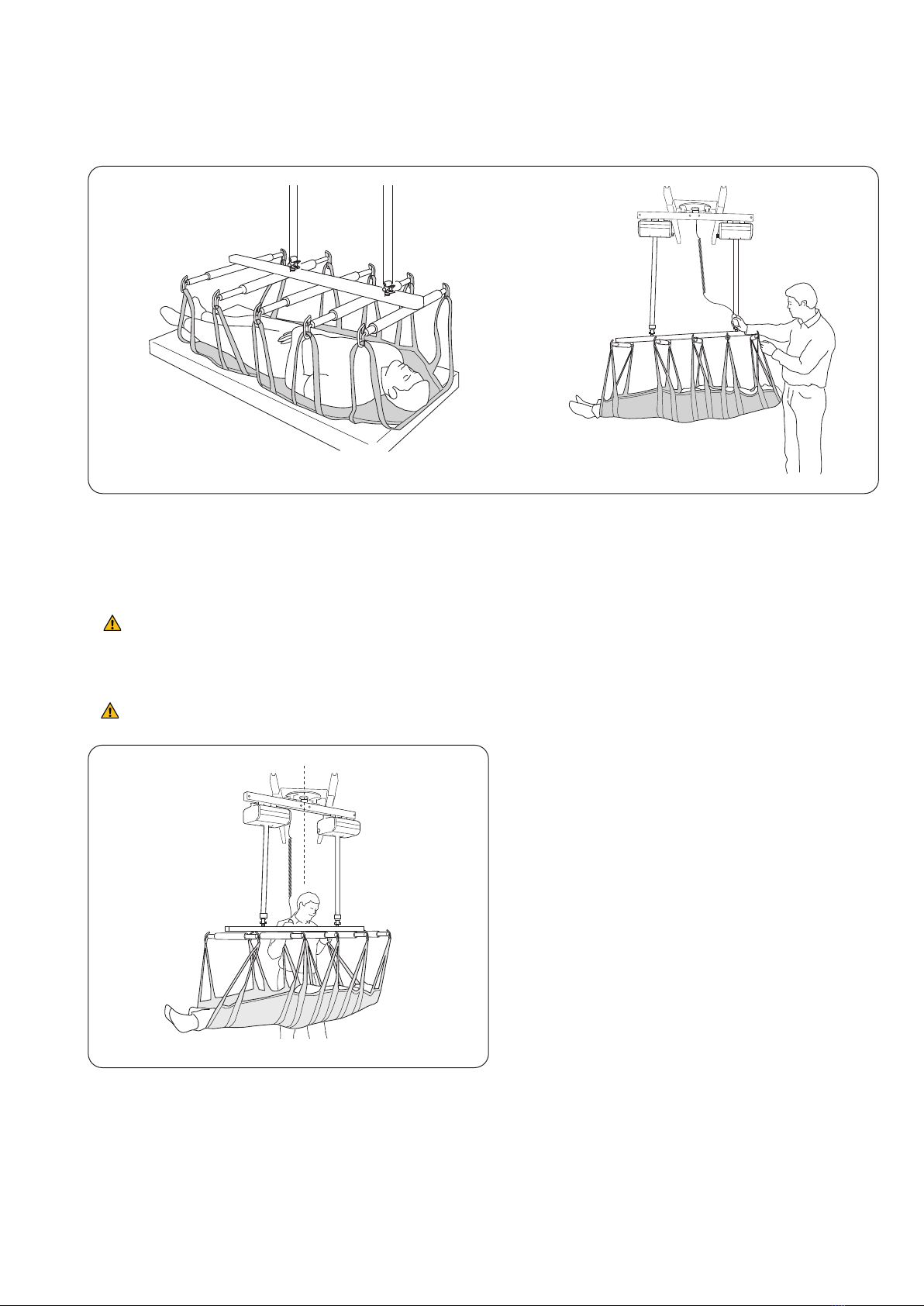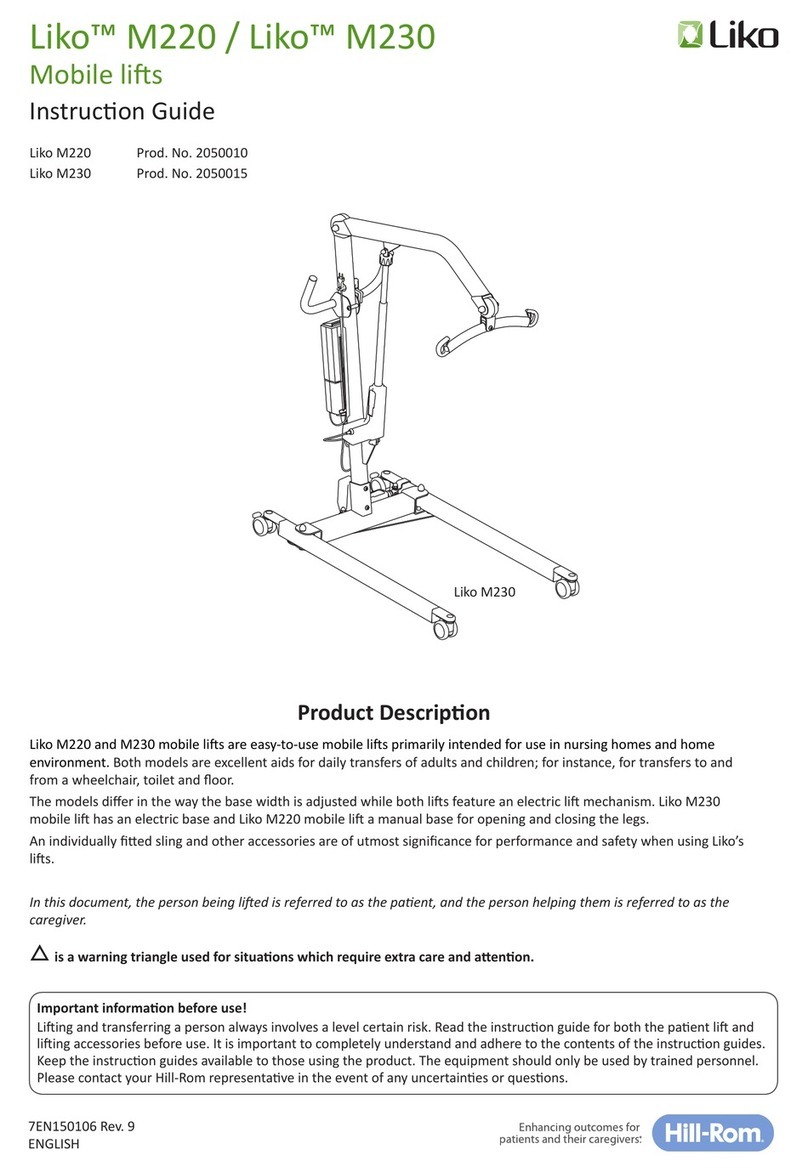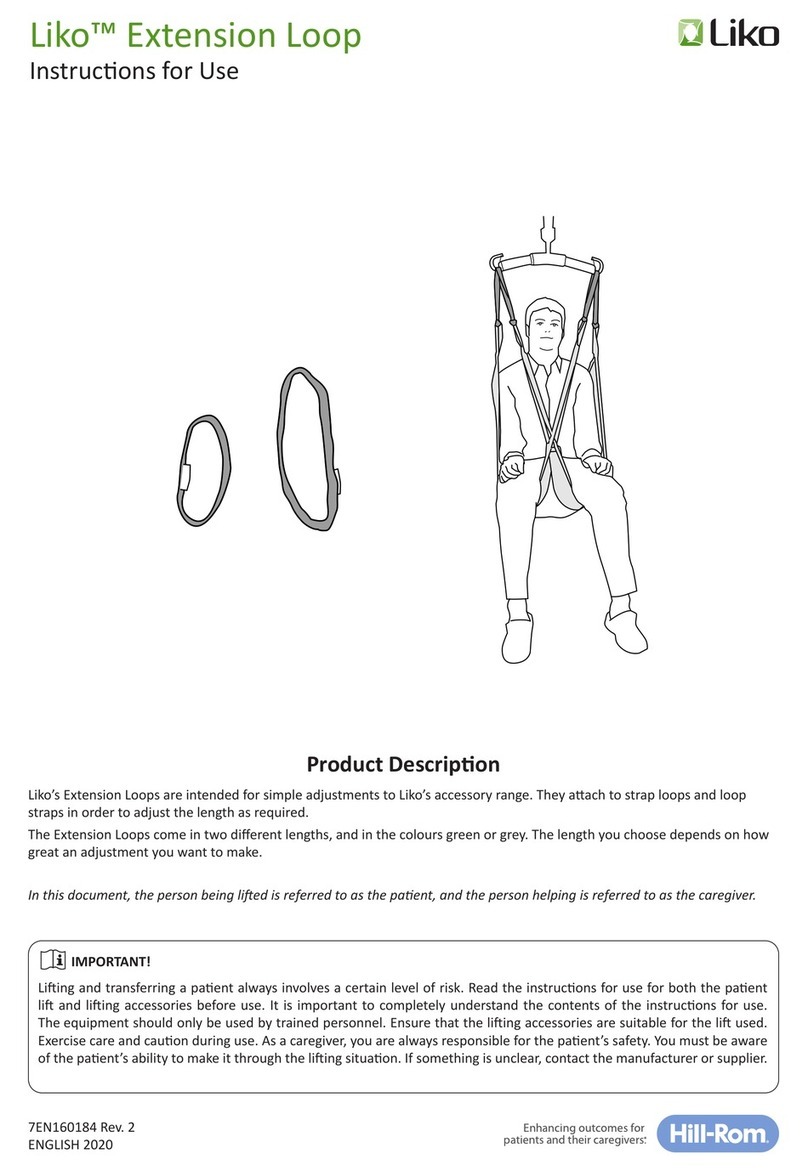
Inspecon and Maintenance
For trouble-free use, certain details should be checked each day the li is used:
• Check the li system for any signs of external damage.
• Check carriage funcon.
• Check the sling bar’s/stretcher’s Quick-release Hook safety funcon.
• Ensure that all screws are ghtened.
When necessary, clean the li with a moist cloth, using warm water or disinfectant. NOTE! Do not use cleaning agents that
contain phenol or chlorine, since these can damage aluminium and plasc materials.
The li should not be exposed to running water.
Service
A periodic inspecon of the UltraTwist should be carried out at least once a year.
Periodic inspecon, repair and maintenance may be performed only in accordance with the Liko service manual by
personnel authorized by Liko and using original Liko spare parts.
Service Agreement
Liko oers the opportunity to enter into service contracts for the maintenance and regular inspecon of your Liko product.
Expected Life Time
The product has an expected life me of 10 years when correctly handled, serviced and periodically inspected in
accordance with Liko’s instrucons.
Transport and Storage
The environment in which the product is transported and stored should have a temperature of 10–40 ˚C (50-104 °F) and a
relave humidity of 30–75 %. The atomspheric pressure should be 700–1060 hPa.
Recycling
Ultra Control Unit should be recycled as Waste of Electrical and Electrical Equipment (WEEE). Electronic parts on UltraTwist
should be recycled as Waste of Electrical and Electrical Equipment (WEEE) and the rest of the product as scrap metal.
Hillrom evaluates and provides guidance to its users on the safe handling and disposal of its devices to aid in the prevenon
of injury, including, but not limited to: cuts, punctures of the skin, abrasions, and any required cleaning and disinfecon of the
medical device aer use and prior to its disposal. Customers should adhere to all federal, state, regional, and/or local laws and
regulaons as it pertains to the safe disposal of medical devices and accessories.
If in doubt, the user of the device shall rst contact Hillrom Technical Support for guidance on safe disposal protocols.
Product Changes
Change to Liko products undergo connuous development, which is why we reserve the right to make product changes
without prior noce. Contact your Hill-Rom representave for advice and informaon about product upgrades.
Design and Quality by Liko in Sweden
The management system for both manufacturing and development of the product is cered in accordance with ISO9001
and its equivalent for the medical device industry, ISO13485. The management system is also cered in accordance with the
environmental standard ISO14001.
Noce to Users and/or Paents in EU
Any serious incident that has occurred in relaon to the device, should be reported to the manufacturer and the competent
authority of the Member State in which the user and/or paent is established.
© Copyright Liko R&D AB 2020. ALL RIGHTS RESERVED.
Liko AB
Nedre vägen 100
975 92 Luleå, Sweden
+46 (0)920 474700
www.hillrom.com
Liko AB is a subsidiary of Hill-Rom Holdings Inc.