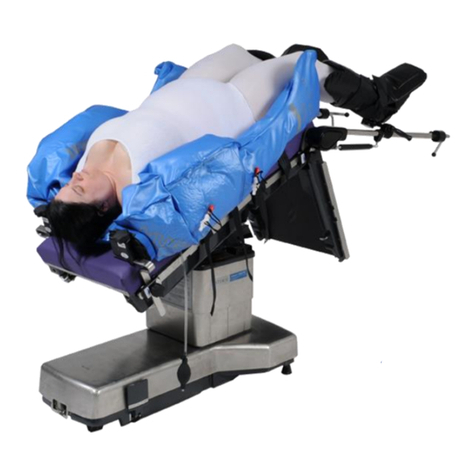
Yellofins Apex™ (O-YFAX)
1.
General Information: .......................................................................................................... 5
1.1
Copyright Notice: .......................................................................................................5
1.2
Trademarks: .................................................................................................................5
1.3
Contact Details:..........................................................................................................6
1.4
Safety Considerations: ...............................................................................................6
1.4.1
Safety hazard symbol notice:.......................................................................6
1.4.2
Equipment misuse notice:.............................................................................6
1.4.3
Notice to users and/or patients:...................................................................6
1.4.4
Safe disposal: ..................................................................................................7
1.5
Operating the system:................................................................................................7
1.5.1
Applicable Symbols: ......................................................................................7
1.5.2
Intended User and Patient Population........................................................9
1.5.3
Compliance with medical device regulations: .........................................9
1.6
EMC considerations....................................................................................................9
1.7
EC authorized representative: ................................................................................10
1.8
Manufacturing Information:....................................................................................10
1.9
EU Importer Information:..........................................................................................10
1.10
Australian sponsor Information: ............................................................................. 10
2.
System................................................................................................................................. 11
2.1
System components Identification: .......................................................................11
2.2
Product Code and Description: .............................................................................12
2.3
List of Accessories and Consumable Components Table: ................................ 12
2.4
Indication for use: .....................................................................................................12
2.5
Intended use: ............................................................................................................13
3.
Equipment Setup and Use: ...............................................................................................14
3.1
Prior to use: ............................................................................................................... 14
3.2
Setup: ........................................................................................................................ 14
3.2.1
Device Controls and Components............................................................14
3.2.2
Initial Setup ....................................................................................................15