( 5 ) Q1E-EP1370
CONTENTS
Page
1. Introduction .................................................1
1.1 General .......................................................... 1
1.2 Principles of operation .......................................... 1
1.3 Intended Use ..................................................... 2
1.4 Components ....................................................... 2
1.5 Option ........................................................... 3
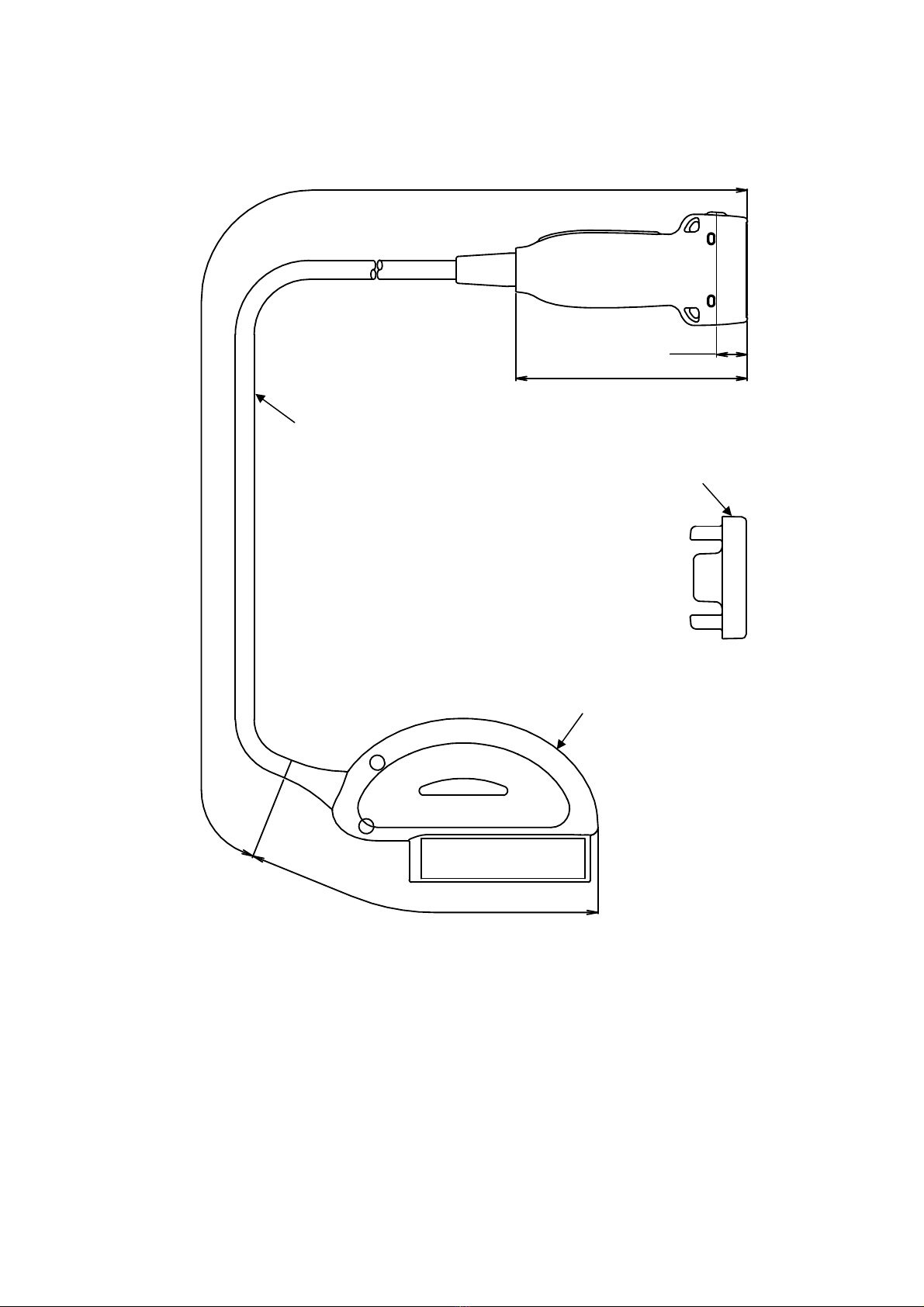
1.6 External View .................................................... 4
2. Inspection before Use ........................................5
2.1 Inspection of appropriate connection ............................. 5
2.2 Inspection of material surface ................................... 5
3. Operation Procedure ..........................................6
4. Option of L44 Probe ..........................................8
4.1 Elasto Coupler ................................................... 8
5. Reprocessing Procedure .......................................9
5.1 Point of use (Pre-cleaning) ..................................... 12
5.2 Containment and transportation .................................. 12
5.3 Manual Cleaning and disinfection ................................ 12
5.4 Drying .......................................................... 16
5.5 Inspection ...................................................... 16
5.6 Packaging ....................................................... 16
5.7 Sterilization ................................................... 16
5.8 Storage ......................................................... 18
6. Maintenance and Safety Inspection ...........................19
7. Safety Precautions ..........................................20
8. Specifications ..............................................21
8.1 Probe ........................................................... 21
8.2 Suppliers List .................................................. 22
9. Disposal of the probe .......................................22