-1-
MN1-6031 Rev. 3
1. General Information
General information for the probe is provided below.
1-1. Intended use
This probe is intended to be used by doctors. The probe is placed in direct contact with human internal organs during
surgery for ultrasonic observation.
Please refer to the ultrasound diagnostic instrument instruction manual used with this probe for the probe intended use
information.
Regarding with the connectable instrument, please refer to section 2-1. Specications of this manual.
Warning
Do not use this equipment for other than its intended use.
Otherwise it could cause burns or other injuries to the patient or operator.
1-2. Classication of ME equipment
This probe is classied as follows according to IEC60601-1.
Please refer to the section 2-1 for the range of applied part, the part treated as applied part, and the range of IPX7.
• Classication based on the degree of protection against electric shock ........ Type BF applied part
• Classication for protection against ingress of liquids .................................. IPX7 (Watertight equipment)
• Operation mode............................................................................................... Continuous operation
• Method of sterilization.................................................................................... Refer to the separate instruction manual
“Cleaning, Disinfection and Sterilization”
1-3. Standard components
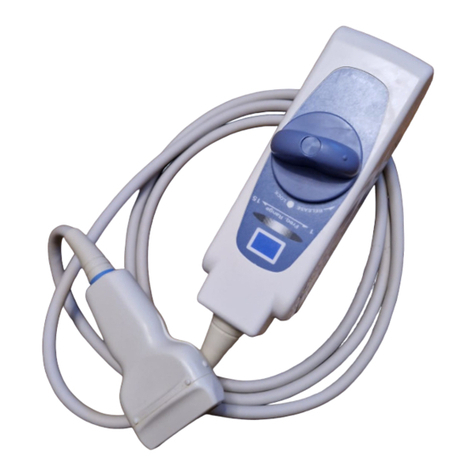
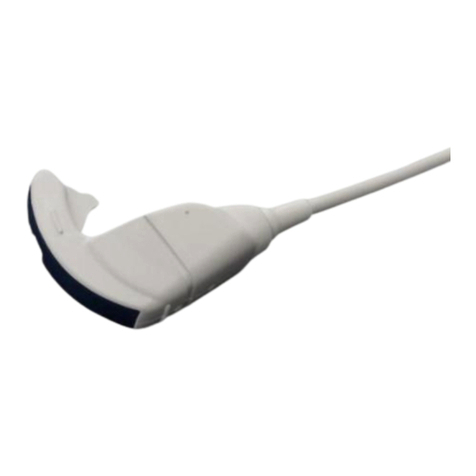
The standard components of C22T probe are as follows.
C22T Probe ··························································· 1 set
Storage case ··························································· 1 set
Instruction Manual
• Specication (MN1-6031) ······································· 1 copy
• Safety (MN1-5984) ··············································· 1 copy
• Cleaning, Disinfection and Sterilization (MN1-6000) ······ 1 copy
1-4. Option
The following options are available for this probe.
• Reprocessing by liquid detergent, disinfectant or sterilant
Whole the probe is able to immerge into the liquids by putting the connector of the ultrasound probe to the
waterproof case WP-001 as below table.
Precautions about the waterproof case, please refer to the instruction manual.
Accessory for reprocessing by liquid detergent, disinfectant or sterilant
Product Name Product No.
Waterproof case WP-001