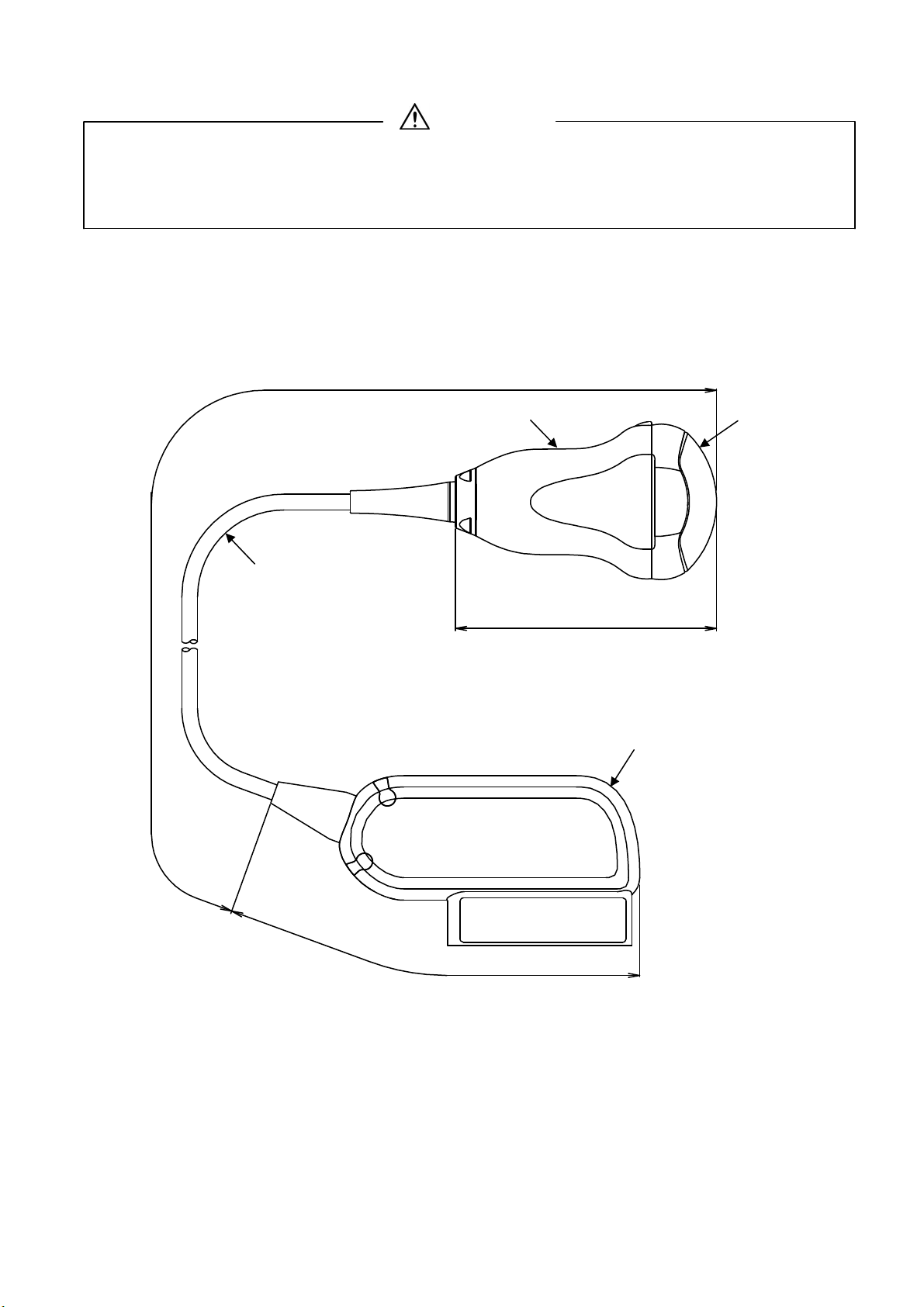
Hitachi VC34A Probe User manual
Other Hitachi Medical Equipment manuals

Hitachi
Hitachi C252 User manual

Hitachi
Hitachi S121 User manual

Hitachi
Hitachi SCENARIA User manual

Hitachi
Hitachi EUP B715 User manual

Hitachi
Hitachi EUP-L65 User manual

Hitachi
Hitachi EUP-L74M User manual

Hitachi
Hitachi S12 User manual

Hitachi
Hitachi UST-9135P User manual

Hitachi
Hitachi EUP-L73S User manual

Hitachi
Hitachi VC35 Probe User manual

Hitachi
Hitachi CC41R1 User manual

Hitachi
Hitachi EUP-B512 User manual

Hitachi
Hitachi EUP-OL334 User manual

Hitachi
Hitachi EUP-VV531 User manual

Hitachi
Hitachi OPTIGEN AP 720STM User manual

Hitachi
Hitachi EUP-C516 User manual

Hitachi
Hitachi UST-678 User manual

Hitachi
Hitachi UST-9124 User manual

Hitachi
Hitachi EUP-C715 User manual

Hitachi
Hitachi L43K User manual