HLD PhysioTouch A02 User manual

USER MANUAL
Ver. A02 SW rev 2.0x
The manual contains instructions for use, maintenance and handling of the device,
as well as safety precautions and warnings. Read through carefully before using
the device, to ensure safety and eciency for you and your patient. Keep for future
reference.

2

3
Table of Contents
1. Safety 5
2. Application specification 7
3. Parts description 9
4. Connectors 10
5. Symbols and product markings 11
6. Installation 12
7. Using the PhysioTouch® - LymphaTouch® device 16
8. Treatment settings 20
9. Maintenance and cleaning 27
10. Troubleshooting 28
11. Service 30
12. Software upgrade 31
13. Disposal 33
14. Technical data 34
15. EMC 36

4
Copyright©
© HLD Healthy Life Devices Ltd. Helsinki, Finland. All material included in this manual is intended
solely for use related to the use of the PhysioTouch® - LymphaTouch® is a trademark of HLD Healthy
Life Devices Ltd.
FDA device class I
Quality ISO9001:2008 / 13485:2003
Manufacturer
HLD Healthy Life Devices Ltd
Kuortaneenkatu 2
00510 Helsinki
Finland
www.physiotouch.com www.lymphatouch.com
Safety and EMC certificate
HLD PhysioTouch® - LymphaTouch® A02 conforms with Medical Device Directive 93/42/EEC and
medical electrical equipment electromagnetic compatibility IEC 60601-1-2.
0537
710359

5
In this User Manual, safety precautions and warnings are indicated with the following signal words.
Make sure to read and understand all safety precautions and warnings before starting to use the
device.
Caution – potentially hazardous situation which, if not avoided, may cause minor to moderate injuries,
or damage to the device.
Warning - potentially hazardous situation which can cause injuries or damage the device, if instructions
are not followed.
Danger – potentially hazardous situation which can result in serious injury or death, if instructions are
not followed.
1. Safety

6
1.1 Safety precautions
• Clean the device and accessories regularly following the instructions and the regulations of your
institution, to ensure safe and hygienic use.
• Use and store the device only under the environmental conditions specified.
• Handle the device with care, and follow the instructions for maintenance.
• Use only accessories and mounting provided by or approved by HLD Healthy Life Devices Ltd.
• Use treatment cups and treatment setting that are best suited to the anatomy and skin type of the
patient you are treating.
• Avoid situations where the unit might fall or collide with a heavy object.
• Inspect the device and the related accessories for cracks or other signs of overuse, and replace
accessories as required.
• Make sure the filter in the treatment cup is in place before attaching it to the Treatment Head, to
avoid dust and debris from being sucked into the device.
• Never use the unit for purposes other than those recommended by HLD Healthy Life Devices Ltd.
HLD Healthy Life Devices Ltd will not be liable for any inappropriate use of the equipment.
• Do not treat directly over skin with open wounds.
• Avoid situations where the cable can be wrap around the neck.
• Do not leave the device in proximity of pets, pests or children.
• This device needs special precautions regarding EMC and needs to be installed and put into
service according to the EMC information provided in Chapter 14

7
2. Application specification
Intended use:
PhysioTouch® - LymphaTouch® is a negative pressure-based therapy device and aid for physiotherapy
and lymphatic drainage therapy. The device is meant to be used by persons who are knowledgeable
about the treatment method or under supervision of those persons.
Intended conditions of use:
The environment, where this device is used typically physiotherapy setting including hospitals,
physiotherapy centres.
Contraindications:
Do not use on a pregnant patient without prior consultation with the attending doctor.
Do not use on a patient with cancer during active treatment period or without prior consultation with
the attending doctor.
Do not use on acute infection.
Be very careful when applying on fragile skin and on the throat area.
Do not use on a patient with a diagnosed current venous thrombosis.
Do not use on a patient suering from protein poor oedema due to severe heart failure, cardiac
oedema.
If you are uncertain of the suitability of the device for treatment of your patient, seek medical advice
prior to starting the treatment.
In addition, all contraindications of physiotherapy and manual lymphatic drainage apply.

8
Intended medical indication:
Improvement of lymphatic circulation in the treated area, improvement of secondary lymphedema and
reduction of secondary lymphedema of the Arm (SLA) Post Mastectomy.
Device is used on any patient deemed to be appropriate for a physical treatment. The patient has no
control of device. Device has no specific age or weight limits on patients.
Operating principle
The mechanism of action is based on the eects of negative pressure in tissues. Negative pressure
created by the treatment device stretches the skin and the tissue underneath, pulling on anchoring
filaments to dilate the endothelial openings of lymph vessels. Vertical stretching of the fascial
(connective tissue) structures is accomplished at the same time, expanding the space for circulation of
blood and lymph. Lymph and the metabolic waste products that impede the healing process can then
flow more easily from the interstitial space into lymph vessels, and excess fluid is carried away by the
body’s own lymph transport mechanisms to re-join the venous circulation.
9
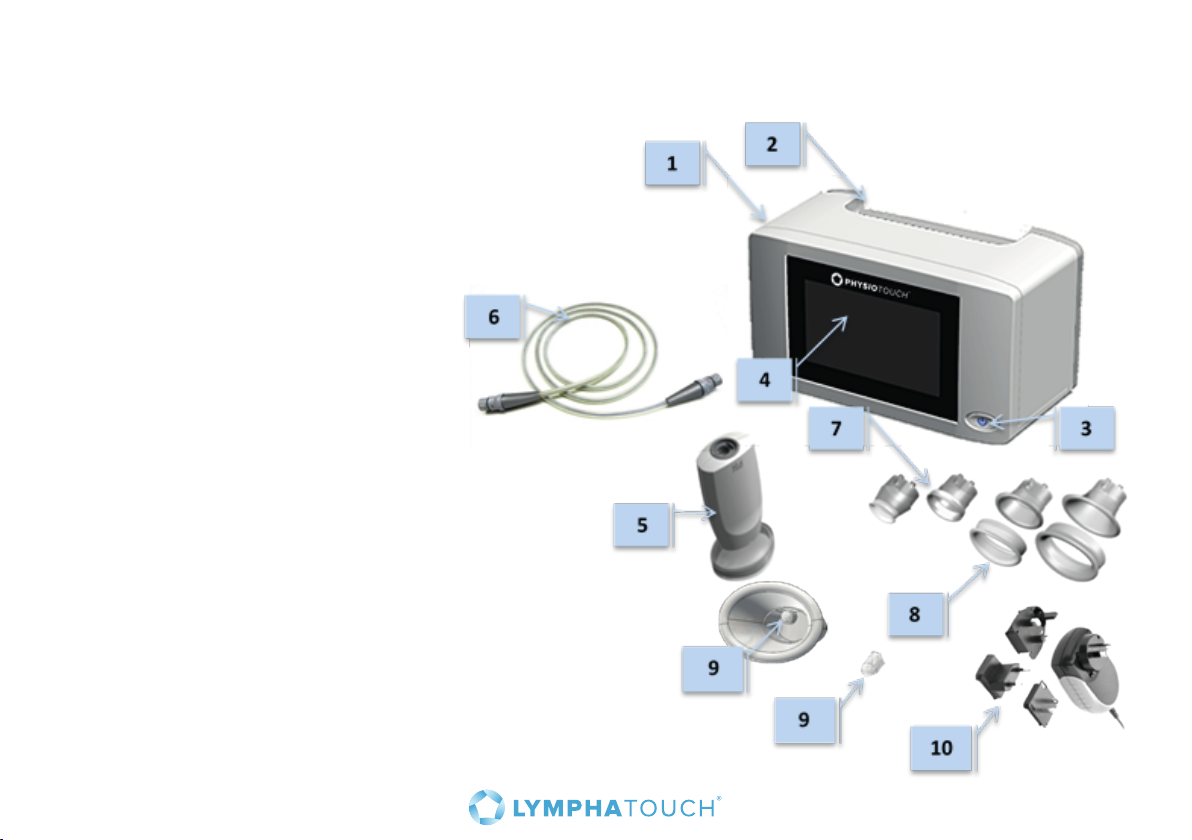
3. Parts description
1. Main unit
2. Handle
3. On / O switch
4. Touch screen
5. Treatment Head
6. Cable
7. Dierent sizes of treatment cups
for the Treatment Head
8. Soft covers for the treatment cups
(detachable on the larger treatment cups)
9. Treatment cup filter
10. DC-power, country adapter included

10
4. Connectors
Warning – Do not touch USB port while treating patient or let the patient touch the USB while being
treated. Do not use USB or LAN connectors while treatment in progress.
1. Device’s serial number
2. Integrated air hose and cable connector
3. Mains power
4. LAN for future use
5. USB
5
2
1
4
3

11
5. Symbols and product markings
In addition to the connector markers described above, you may find the following symbols and
markings in the device:
Symbol Description
0537 The product is CE-marked and the number indicates the notified body.
Read the User Manual before using the device.
The device is classified as Class BF, according to the standard IEC 60601.
The device has double insulation; connection to mains does not require a grounding pin.
Special instructions are needed to dispose device properly.
Lithium-ion battery must be disposed properly.
The carton package of the device can be recycled.
This side up.
Weights less than 5.51 lbs.
Do not use sharp instrument to open the package
Fragile. Handle with care.
Humidity limitation. See more from the table page 34.
Keep dry.
Temperature limitation. See more from table page 34.

12
6. Installation
6.1 Unpacking
Carefully unpack all parts of the device and confirm that all parts are included and intact.
Caution - We recommend you to keep the box in case you need to return the device
or send the device for service – it is most safely transported in the box it was originally
shipped in.
6.2 Internal Battery
PhysioTouch® - LymphaTouch® has internal battery which is advised to charge full before
first usage.
During battery recharge, amber light blinks in the power button. When battery is fully
charged amber light is on constantly. When device is used via battery and the device is
on, the light is green.

13
6.3 Connecting and disconnecting the cable
Attach the integrated air hose and cable to the round connector on the left side of the
unit. Make sure the cable is tightly connected, but do not use excessive force or rotate
connector. To disconnect, pull the connector straight.
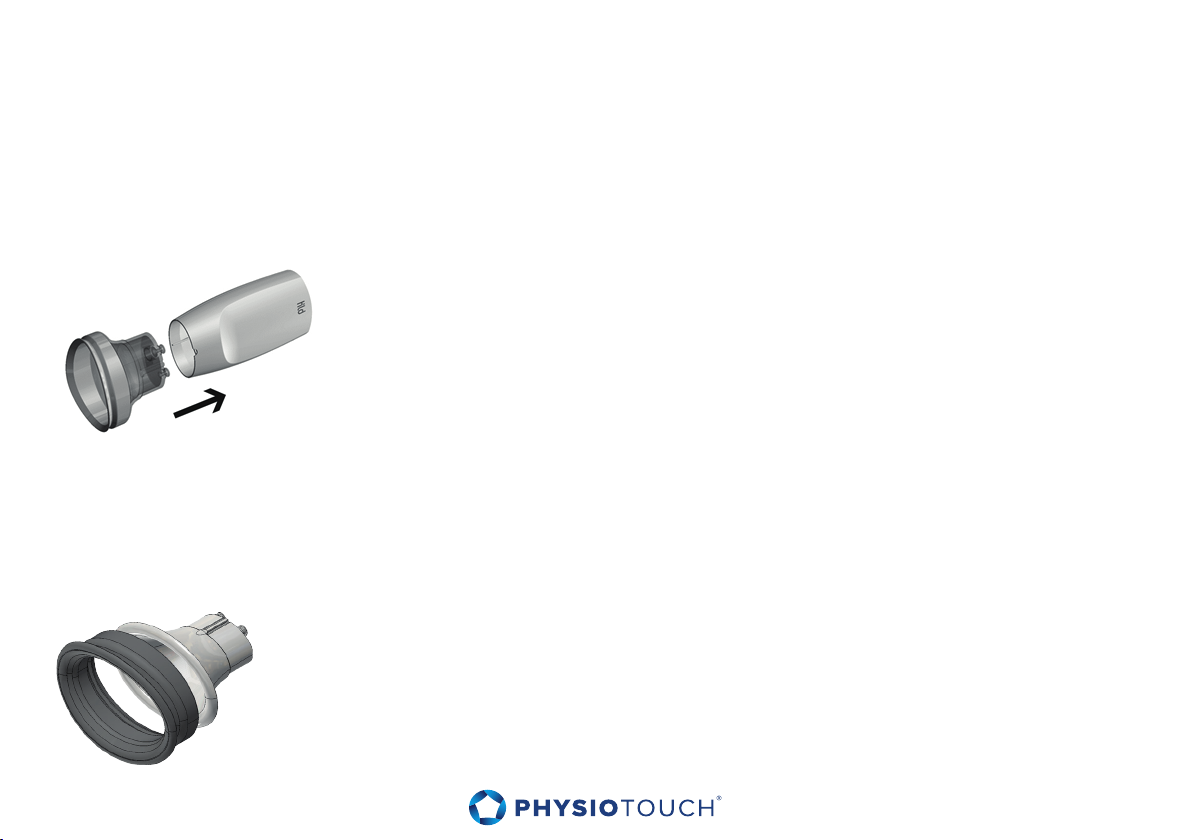
6.4 Connecting and disconnecting the Treatment Head
Attach the integrated air hose and cable to the round connector on the end of the
Treatment Head. Make sure the cable is tightly connected, but do not use excessive
force or rotate connector. To disconnect, pull the connector straight.
14
6.5 Connecting a treatment cup to the Treatment Head
Select the appropriate treatment cup for the patient and body area in question. Please
refer to the Chapter 7.4 ”Selecting a treatment cup”.
Connect the treatment cup to the Treatment Head as indicated in
the figure.
Caution - Make sure the treatment cup is connected in right orientation, as indicated by
the guiding rails.
6.6 Connecting removable soft part to the treatment cup
60mm and 80mm treatment cups have separate soft parts. Connect the
treatment cup soft part to treatment cup as indicated in the figure.

15
6.7 Placing the PhysioTouch® - LymphaTouch® device for treatment use
We recommend mounting the PhysioTouch® - LymphaTouch® to the Roll Stand
(see figure) when the device is used primarily in one location. The Roll Stand is
designed for safe, ecient and ergonomic use.
Instructions for connecting the PhysioTouch® - LymphaTouch® main unit to the
Roll Stand are included in the Roll Stand package.
Caution – Use only mounting elements provided or recommended by HLD
Healthy Life Devices Ltd to mount the device. If the device is not attached
properly, it may fall and cause injury or damage to the equipment.
Caution – HLD0901 Roll stand is designed solely to be used with PhysioTouch®
- LymphaTouch® device. Do not connect other devices or equipment to the
Roll stand. Basket of the roll stand is designed to withstand the weight of 4
treatment cups and the treatment head. Do not exceed this limit.
Caution – If used without Roll stand place the device on a flat surface and be
sure that the device is securely on top of the surface and device can’t falll o.

16
7. Using the PhysioTouch® - LymphaTouch® device
7.1 Turning the device on and o
7.2 Screen saver
Turn the device on by pressing the on/o switch in the lower right hand corner. Device starts in 35
seconds. To turn o the device, press the on/o switch. The device will also shut down automatically
after an hour of inactivity, while on battery power.
The display will shut down and the device will enter a power-saving mode when the device has not
been used for 30 minutes. Reactivate the unit by pressing the on/o switch or by touching the screen.

17
7.3 Patient preparation
PhysioTouch® - LymphaTouch®–treatment does not require special preparation of the patient. The
treatment is applied to bare skin.
Moderate application of oil or massage cream may allow the treatment cup to glide more smoothly on
the skin. Avoid excessive use of oil or massage cream.
If the patient’s skin is very dry and producing dandru, the filter in the treatment cup may get clogged
faster than normal. This will result in diculty achieving the set pressure. Replace the treatment cup in
order to continue an ecient treatment. The error message below is displayed if filter is clogged. Do
not use talcum powder or other powdery top agents on the skin.

18
7.4 Selecting a treatment cup
Select the proper size treatment cup for each treatment situation. Please refer to the table below for
general guidance, while using your own clinical judgment as to what is an appropriate size for each
situation.
Treatment cup Size Removable rubber ring Body areas
HLD0335
35 mm
• Local scars
• Small joints
• Face
• Head and neck
HLD0350-2
50 mm
• Local scars
• Joint areas
• Face
• Head and neck
• Palm area
HLD0360
60 mm X
• Whole body
HLD0380
80 mm X
• Torso
• Upper and lower
limbs
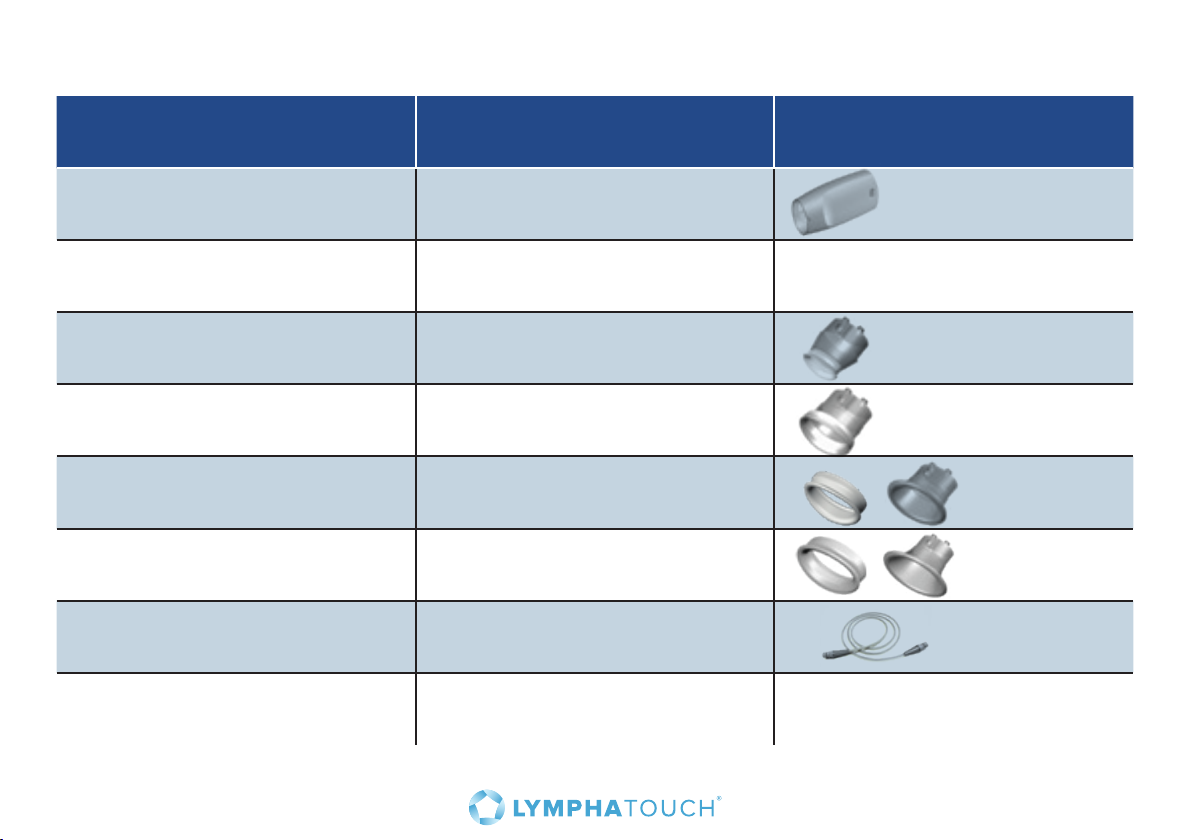
19
Code Accessory
HLD1300 Treatment head
HLD0901 Roll Stand See page 15.
HLD1020-35 Treatment cup 35mm, 20pcs
HLD1020-50 Treatment cup 50mm, 20pcs
HLD1020-60 Treatment cup 60mm, 20pcs
HLD1020-80 Treatment cup 80mm, 20pcs
HLD0122 Treatment Cable
HLD1601 PhysioTouch® - LymphaTouch®
carrying bag
7.5 Accessories
Use these codes to order accessories from the nearest distributor.
20
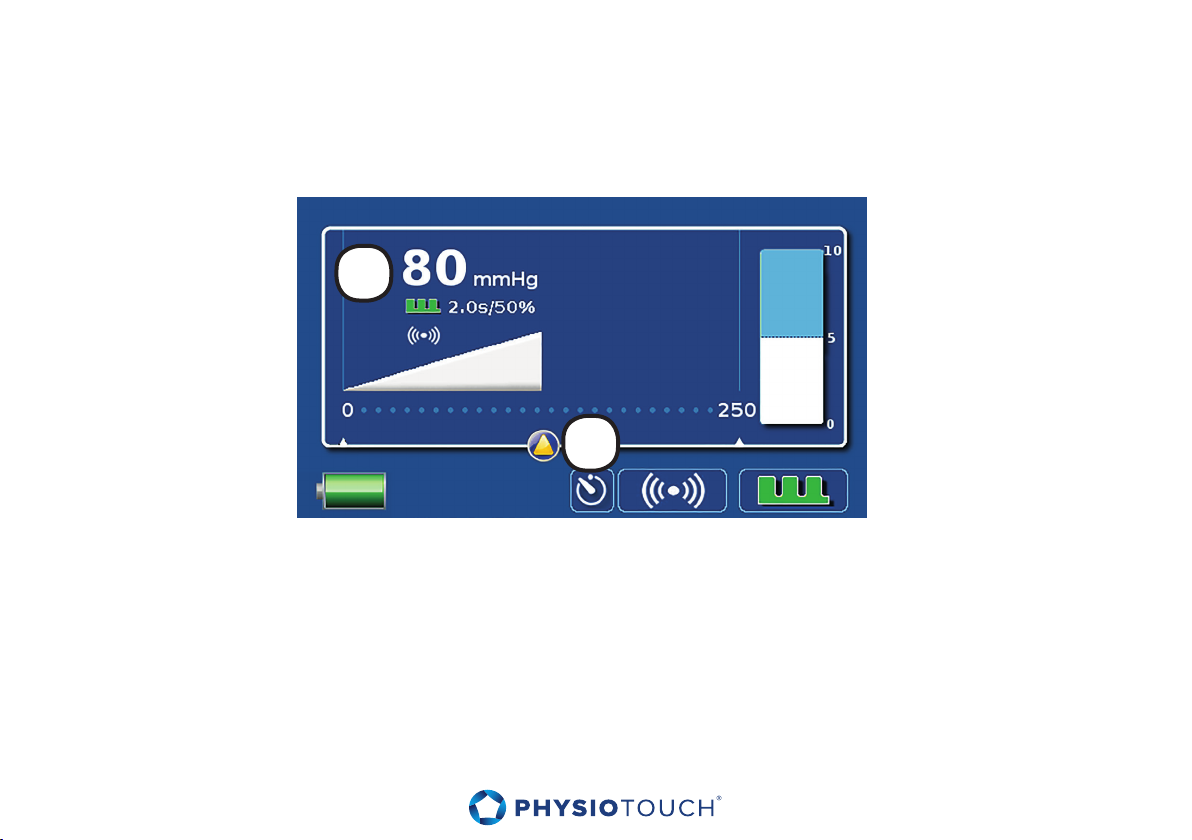
8. Treatment settings
Turn on the device. PhysioTouch® - LymphaTouch® main screen will appear (see picture).
The default setting is 80 mmHg with 2 s. pulsations. The unit will start operation automatically when
the treatment cup is put close to the treated target.
1. The setting is indicated on the main screen on the top left, as well as graphically in the form of
a wedge. During treatment a blue wedge appears which indicates the current vacuum in the
treatment cup.
2. The vacuum is adjusted with the touch screen by moving the slider at the desired location.
If the desired vacuum is not achieved make sure that the treatment cup selected is suitable to the
aected area of the body. If necessary replace the treatment cup with a more appropriate size, or
reposition the treatment cup on the skin carefully. Also, the patient’s body hair may cause air leakage
from the treatment cup. Refer to the section ”Troubleshooting.”
1
2
This manual suits for next models
1
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











