Page 3 Instructions for Use: HotDogTM Head Warming Wrap
INSTRUCTIONS FOR USE
1. Inspect the surface of the Head Warming Wrap for damage (e.g., cuts, holes, loose electrical
connections). Do not use the Head Warming Wrap if it is damaged.
2. Place the Head Warming Wrap around the patient’s head.
Warning: DO NOT place the heated area of the Head Warming Wrap under the patient.
3. Insert one end of the electrical cable into the appropriate port on the HotDog Controller.
4. Insert the other end of the cable into the electrical connector on the Head Warming Wrap.
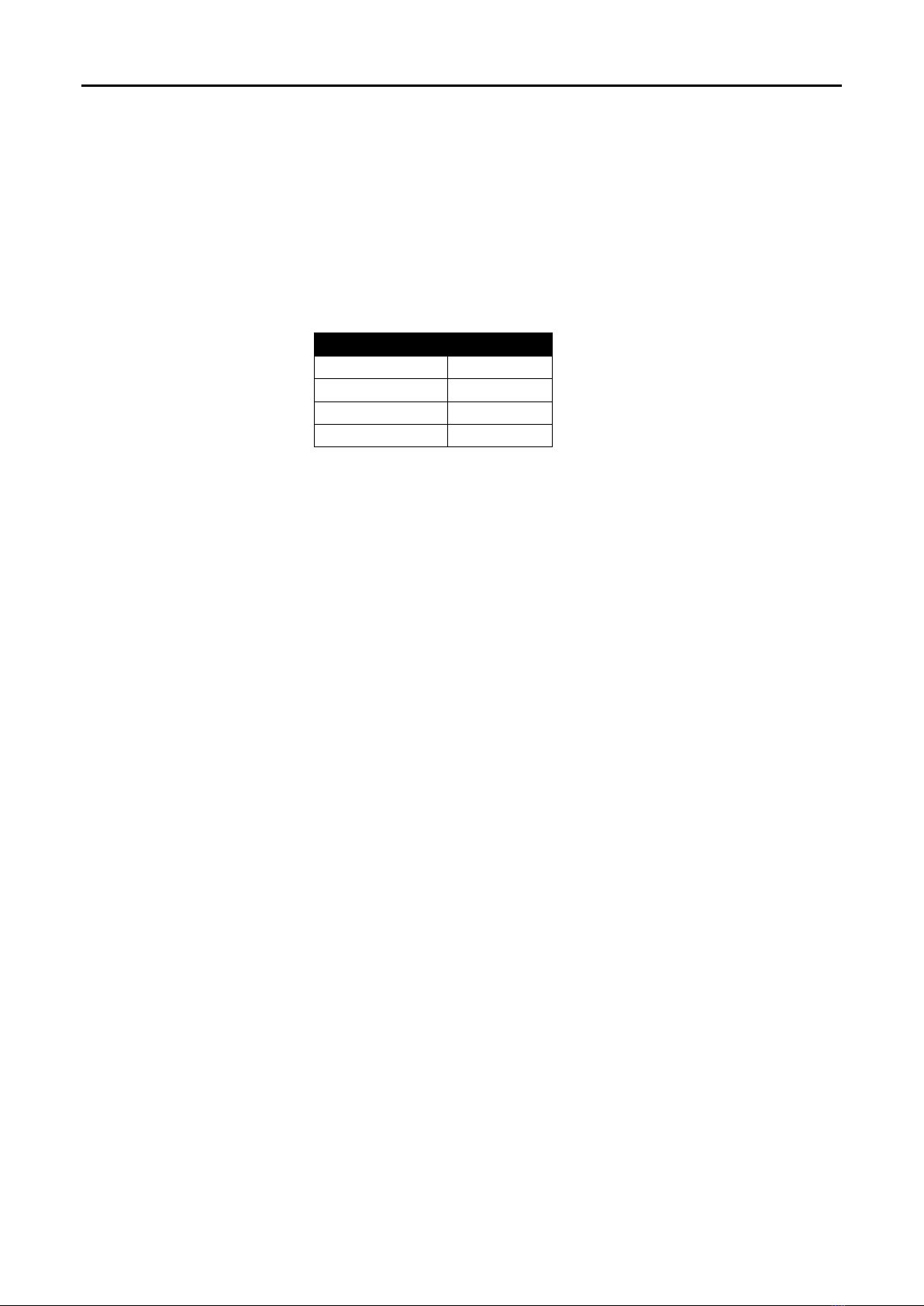
5. Turn the HotDog Controller on and select the desired temperature setting to begin warming. The time
to reach the set-point temperature from 23 C +/-2 C is less than 10 minutes. If the Head
Warming Wrap does not reach the selected temperature within 10 minutes an alarm will sound (Refer
to the HotDog Controller User Manual.)
6. If the HotDog Controller alarm sounds when the Head Warming Wrap is connected, do not use the
Head Warming Wrap until the alarm condition is resolved. (Refer to the “Alarms” section.)
7. When use of the Head Warming Wrap is complete, clean the Head Warming Wrap as necessary. (See
“Care and Maintenance” section below.)
CARE AND MAINTENANCE
DO NOT continue to use the Head Warming Wrap beyond the labeled expiration date.
DO NOT launder or sterilize as this may damage the Head Warming Wrap.
DO NOT immerse the Head Warming Wrap in liquids.
DO NOT use high-level disinfectants (e.g. gluteraldehyde and peracetic acid) or hydrogen peroxide-based
solutions to clean the Head Warming Wrap.
DO NOT spray cleaning solutions into the electrical connector.
DO NOT use cleaning or disinfection methods different from those recommended in the Instructions for
Use without first checking with an authorized service representative to ensure that the proposed methods
will not damage the equipment.
DO NOT use the Head Warming Wrap if it shows signs of damage or excessive wear such as cuts, holes,
or loose electrical connectors. Technical staff should perform inspection, such as electrical leakage and
resistance testing, to determine if it is safe for use.
DO NOT disassemble the Head Warming Wrap; the product contains no serviceable parts. If service is
required, call an authorized service representative for assistance.
Storage
Store the Head Warming Wrap in a dry place and in a manner that prevents it from being cut or crushed.