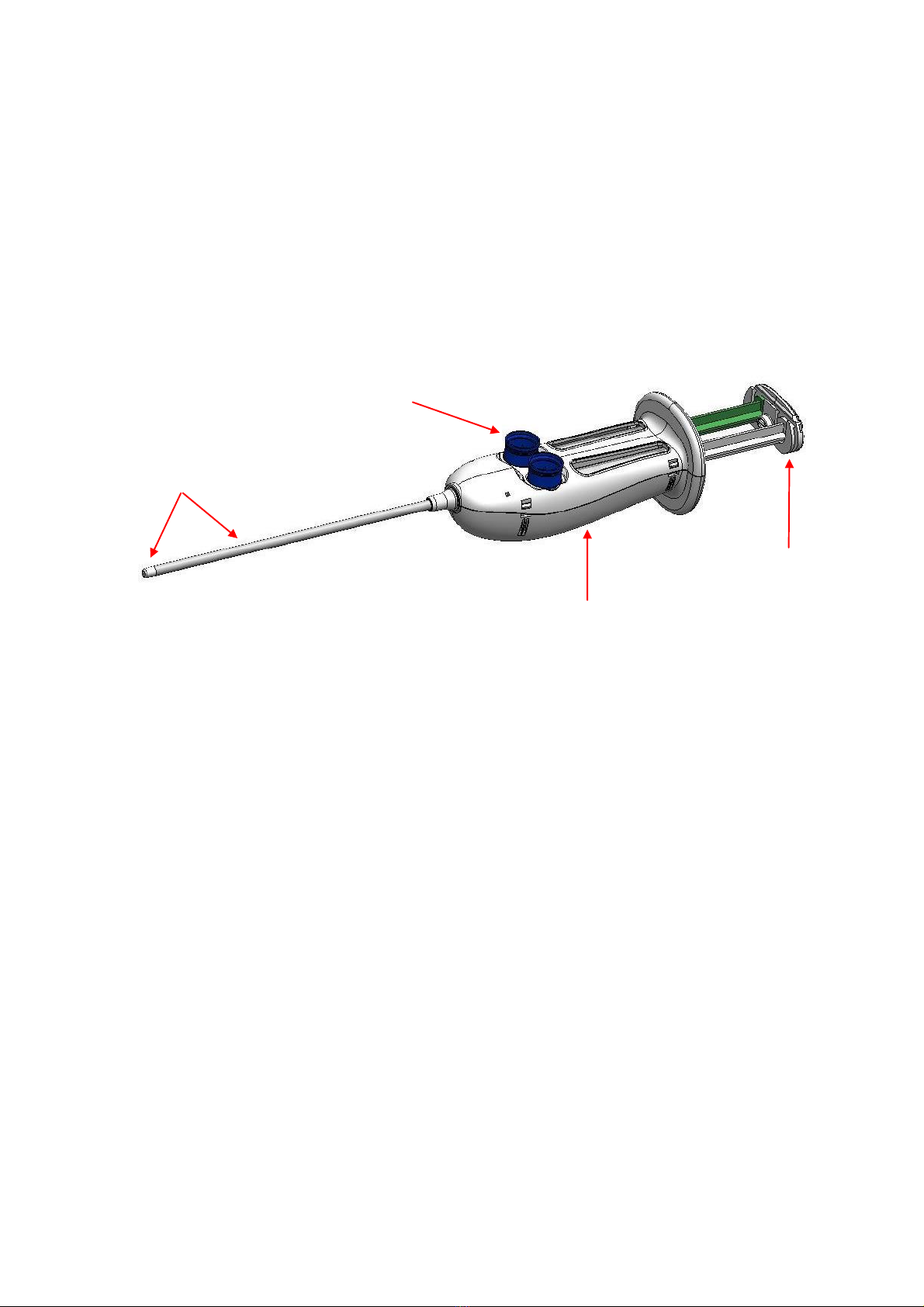
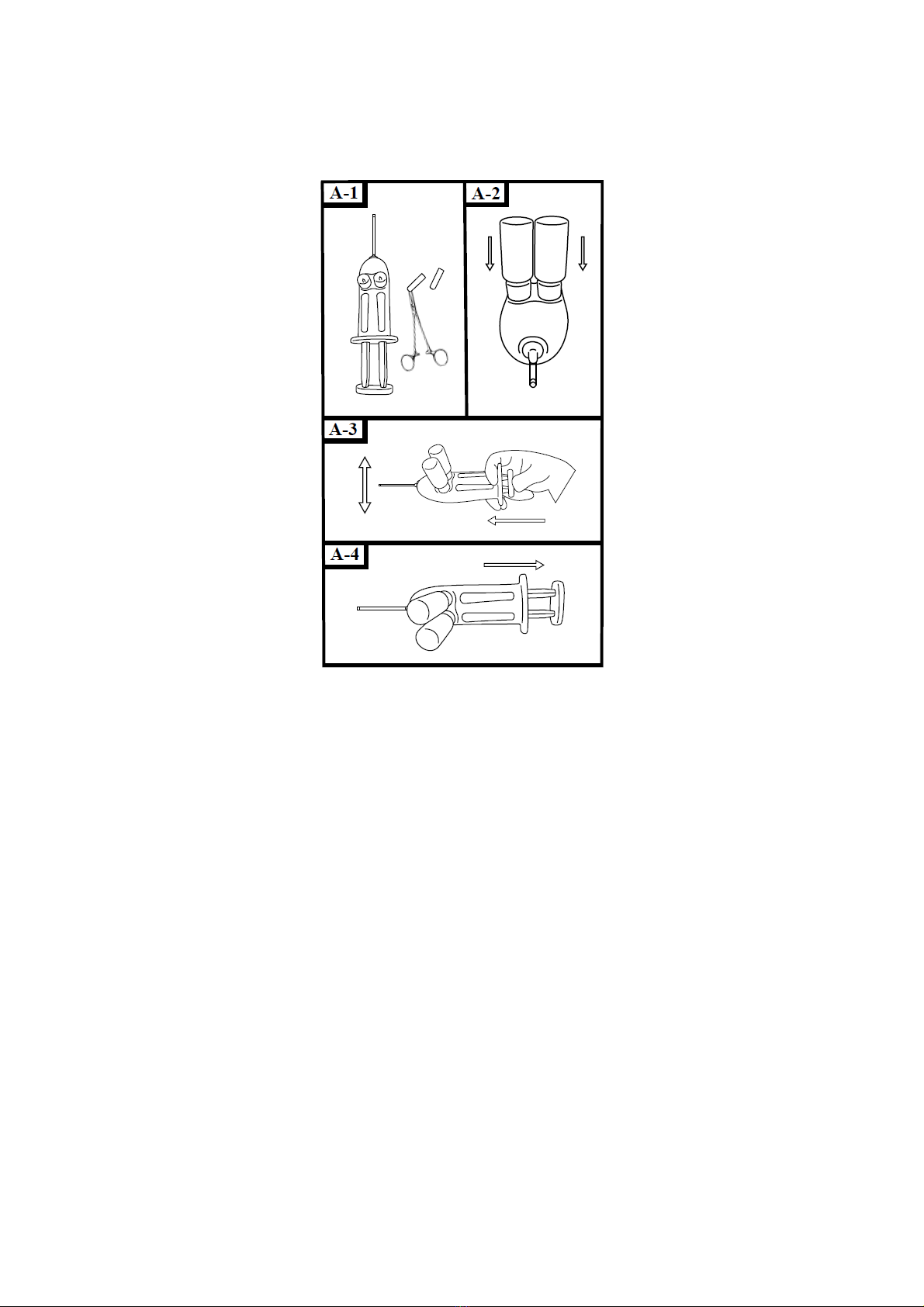
7
retreatment of the original surgical site within 120 days post-surgery. The overall success rate for the complete
case analysis was 91.2% in the Adherus group compared to 90.6% in the control group. Adherus was found to be
non-inferior to the control with the non-inferiority margin of 10% (p = 0.005). In the early post-surgical period when
the sealants are expected to function, the overall success rate at the 14-day follow-up on subjects who completed
the visit was 99.1% in the Adherus group compared to 95.0% in the control group. In addition, the overall success
rate at the 45-day follow-up on subjects who completed the visit was 96.6% in the Adherus group compared to
91.9% in the control group. The freedom from primary endpoint failure analysis also showed that the primary
endpoint failures in the control group tended to occur early in the follow-up period while a majority of Adherus
endpoint failures were identified through protocol-required radiographic imaging at the 120-day follow-up visit.
Safety was assessed based on evaluation of wound healing, surgical site infections, meningitis, worsening
Modified Rankin Score, surgical site swelling and adverse events through the 120 day follow-up period. One
hundred (80.6%) subjects in the Adherus group and 98 (77.8%) subjects in the control group experienced at least
one adverse event within the 120 day follow-up period. There were no unanticipated adverse device effects.
Among the subjects treated with Adherus AutoSpray Dural Sealant, there were no device related deep wound
infections and no cases of meningitis. The type and rate of adverse events observed in this study are consistent
with the complexity of the surgical procedure and the co-morbid condition of the treated subjects.
Key Inclusion/Exclusion criteria for the study included the following:
Pre-Operative Inclusion Criteria:
Subject was ≥ 18 and ≤ 75 years of age.
Subject was scheduled for an elective cranial procedure involving a dural incision using any of the
following surgical locations / approaches (or combination): frontal, temporal, occipital and parietal (i.e.
supratentorial), and/or midline or lateral suboccipital (i.e. infratentorial).
Subject required a procedure involving a Class I/clean wound (uninfected surgical wound in which no
inflammation was encountered).
Pre-Operative Exclusion Criteria:
Subject required a procedure involving a translabyrinthine, transsphenoidal, transoral approach, or any
procedure that penetrates the air sinus or mastoid air cells. Note: Superficial penetration of mastoid air
cells was not an exclusion if cells were appropriately sealed (e.g. bone wax).
Subject had a CSF shunt such as; ventriculo-peritoneal, ventriculo-pleural, ventriculo-atrial or lumbo-
peritoneal shunts.
Subject had an external ventricular or lumbar CSF drain that must be left in place after surgery.
Subject had clinically significant hydrocephalus or clinical evidence of altered CSF dynamics.
Subject had undergone a previous intracranial neurosurgical procedure in the same anatomical location.
(Note: stereotactic biopsy was not exclusionary).
Subject experienced previous CSF leak (secondary to trauma, neoplasm, surgery or other etiology).
Subject had radiation treatment to the surgical site, or standard fractionated radiation therapy was
planned within ten days post index-procedure. (Note: stereotactic radiosurgery prior to the planned index
procedure was not an exclusion criterion).
Subject had experienced a traumatic injury to the head within 30 days prior to the planned index
procedure resulting in basilar skull fracture or fractures involving the paranasal sinuses.
Subject had a known malignancy or another condition with anticipated survival shorter than six months.
Subject had undergone chemotherapy treatment, excluding hormonal therapy, within three weeks prior to
the planned index procedure, or use of intracavitary chemotherapy wafer (BCNU) was planned, or
chemotherapy treatment was planned within two weeks after the index procedure was performed.
Standard use of peri-operative steroids (i.e., corticosteroids) was permitted. Chronic steroid use (defined
as daily use of corticosteroids for >/= 8 weeks) for the purposes of reducing the side effects of
chemotherapy and/or radiation therapy for cancer was not exclusionary unless the patient was deemed
by the investigator to be suffering from steroid toxicity. Use of corticosteroids on a chronic basis (as
defined previously) for purposes other than decreasing the symptoms of systemic chemotherapy was
exclusionary unless those steroids were discontinued 4 weeks prior to the planned index procedure.
Subject received warfarin, heparin, other anticoagulant agents, aspirin or non-steroid anti-inflammatory
agents on a daily basis and pre-surgical, standard of care drug wash-out did not occur.
Subject had a documented clinically significant coagulopathy with a PTT > 37 seconds, or INR > 1.3 INR
units.
Subject had a compromised immune system or autoimmune disease, or was on chronic
immunosuppressant agents at baseline.
Subject had a systemic infection or evidence of any infection near planned operative site.
Subject had a serum creatinine level > 2.0 mg/dL.
Subject had a serum total bilirubin > 2.5 mg/dL at baseline.