Icare HOME Patient Guide ENGLISH
Icare HOME Patient Guide ENGLISH
Icare HOME (Model: TA022) PATIENT
GUIDE TA022-035
TABLE OF CONTENTS
Safety instrucons................................................ 3
Glossary................................................................
Introducon..........................................................
How does the tonometer work ............................
Contraindicaons .................................................
Risks .....................................................................
Benefits ................................................................
Tonometer overview ............................................
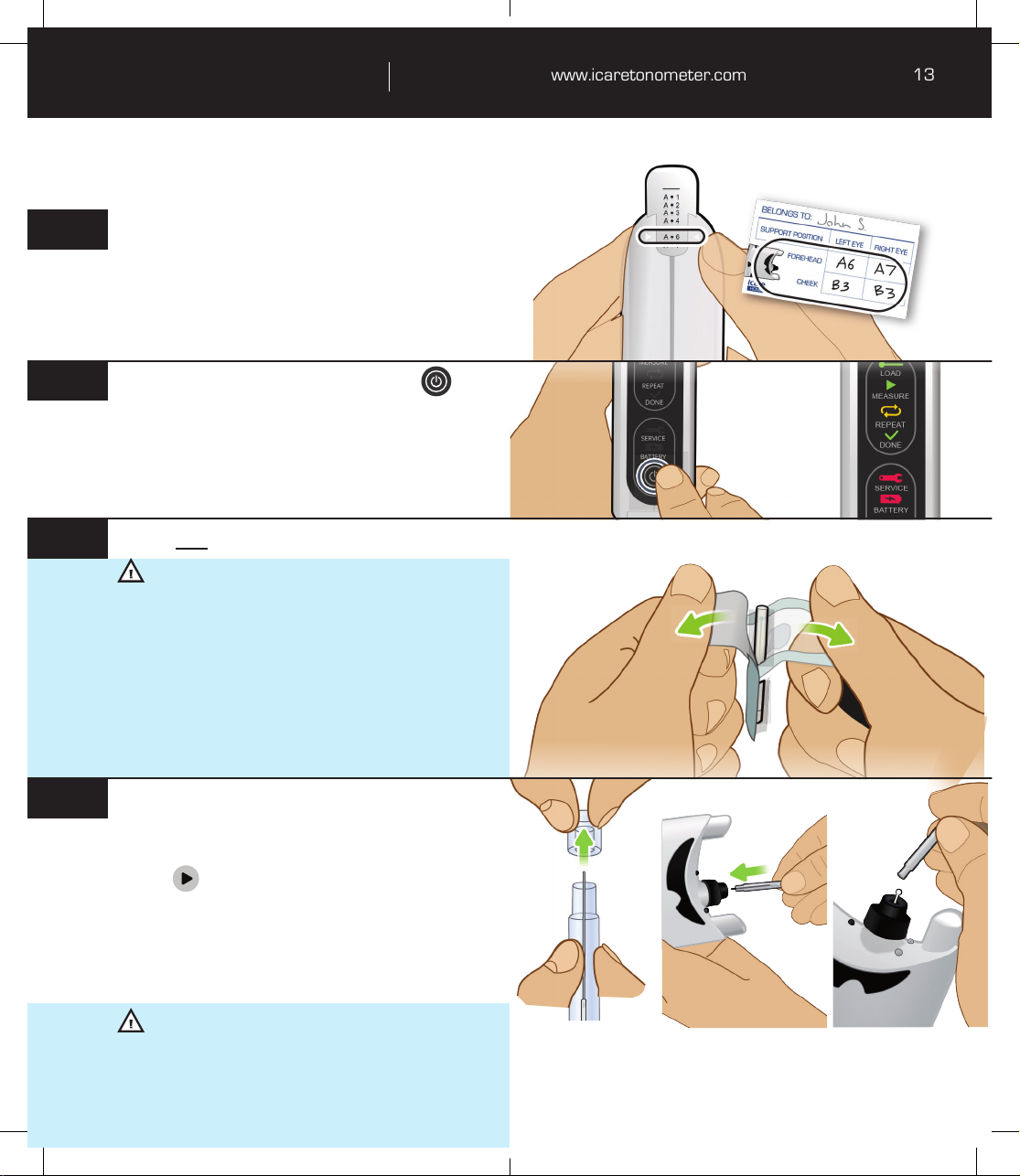
Preparing the tonometer for use .........................
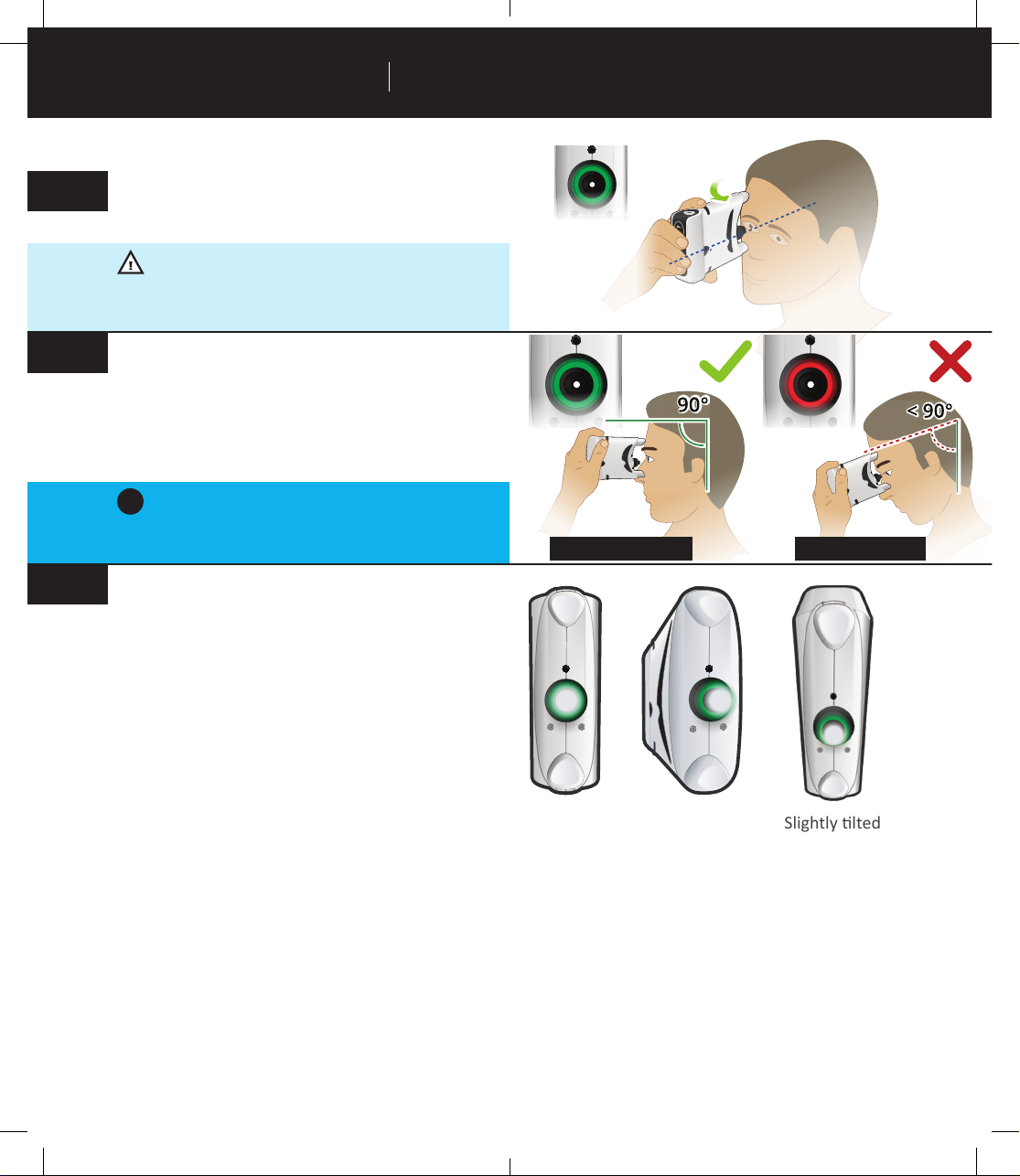
Posioning the tonometer ...................................
Compleng the measurement .............................
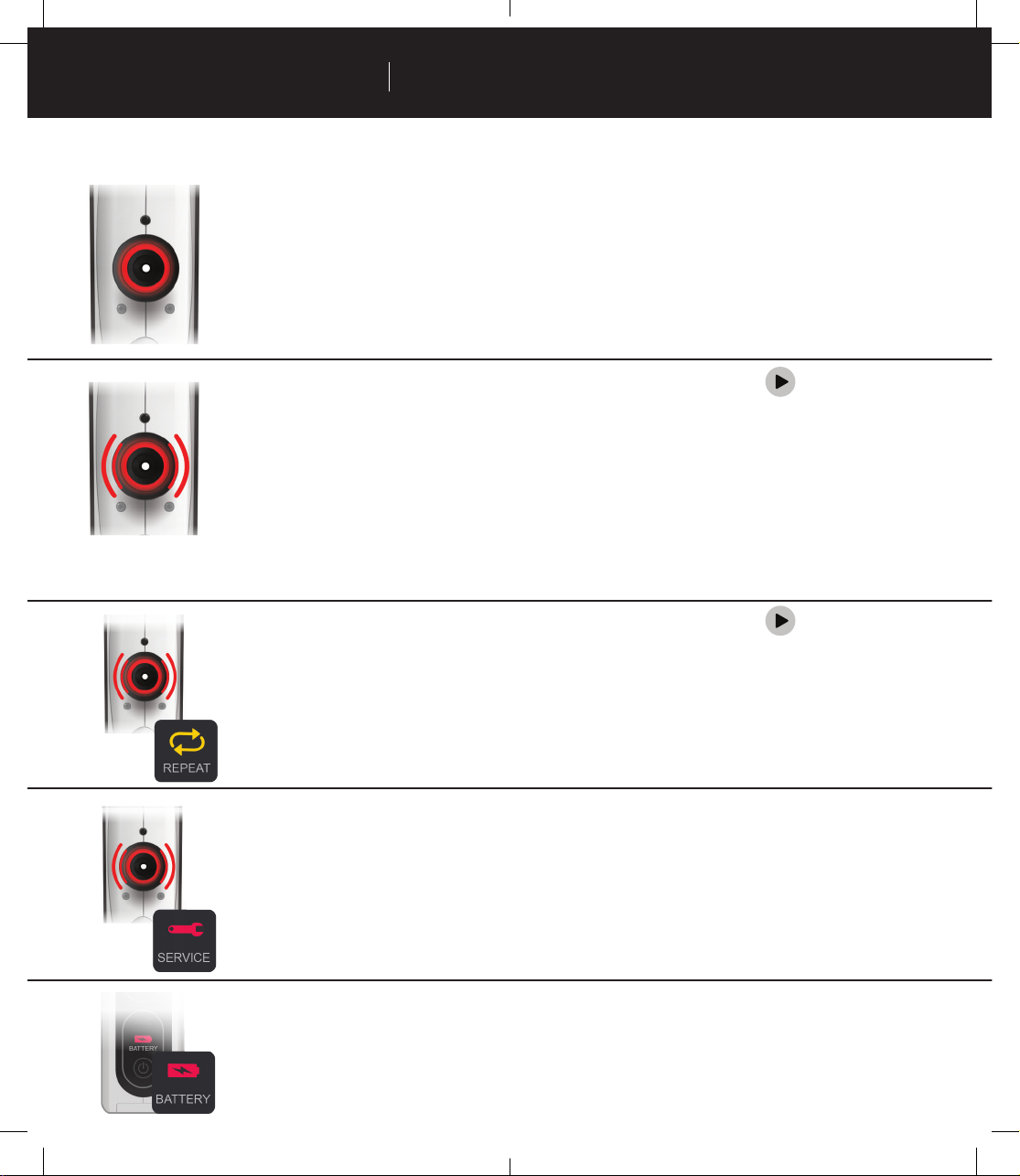
Troubleshoong ..................................................
Replacing the baeries ........................................
..................................... 20
..................
........................... ......
............................................................. .
SAFETY INSTRUCTIONS
WARNING!
WARNING!
WARNING!
WARNING!
WARNING!
WARNING!
WARNING!
WARNING!
D
WARNING!
WARNING!
WARNING!
Icare Finland Oy Äyritie
22, 01510 Vantaa
Copyright © 2019 Icare Finland Oy
Made in Finland
0598
................................................... ...
................................................................ 2
..............................
.................................................... 31
SAFETY INSTRUCTIONS
WARNING!
You should not use the device in areas with a high noise level where
sound error codes cannot be heard.
WARNING!
Do not push the tonometer into the eye (never use a probe that has
no plasc p).
WARNING!
Keep the tonometer out of the reach of children and pets, because
the probe base, baery compartment cover and probes are so small
that a child or pet could swallow them.
WARNING!
The probes are for single-use only, and are packaged sterile.
WARNING!
To prevent contaminaon, do not touch the bare probe, do not use
a probe if it touches a non-sterile surface like a table or a oor.
WARNING!
Do not to modify or disconnue your treatment plan without
receiving instrucons from the health care professional.
WARNING!
For cybersecurity do not connect anything to the USB port except
when uploading paent measurement data. All other tradional
cybersecurity controls (an-virus soware, malware soware,
separate network for the device, etc.) do not apply since the device
is stand alone, is not networked, and does not contain operang
system soware.
WARNING!
Do not change the baeries or probe base when the USB cable is
connected.
WARNING!
Do not connect the USB cable during measurement, the tonometer
will not allow you to take measurements while the USB cable is
connected.
WARNING!
No modicaon of this equipment is allowed.
WARNING!
Use only the original and cered probes made by the
manufacturer. The probes are for single-use (single pair of
measurement sequences) only. Use probes taken only from the
intact, original packaging. The manufacturer cannot guarantee
sterility of the probe once the seal is compromised. Re-sterilizaon
or re-use of the probe could result in incorrect measurement values,
in the breakdown of the probe, cross-contaminaon of bacteria or
viruses, and infecon of the eye. Re-sterilizaon or re-use will void
all resposibilies and liabilies of the manufacturer concerning the
safety and eecveness of the tonometer.
Icare HOME (Model: TA022) PATIENT
GUIDE TA022-035-EN-4.0
This device complies with:
Medical Device Directive 93/42/EEC
Canadian Medical Device Regulations
RoHS Directive 2011/65/EU
Copyright © 2019 Icare Finland Oy
Made in Finland
Icare Finland Oy Äyritie
22, 01510 Vantaa
Finland
TABLE OF CONTENTS
Safety Instrucons....................................................3
Glossary....................................................................7
Introducon..............................................................7
How does the tonometer work ................................7
Contraindicaons .....................................................8
Risks..........................................................................9
Benets ....................................................................9
Tonometer overview ..............................................10
Preparing the tonometer for use............................12
Posioning the tonometer .....................................14
Compleng the measurement ...............................16
Troubleshoong .....................................................18
Replacing the baeries...........................................20
Replacing the probe base .......................................20
Cleaning the tonometer .........................................22
Transferring the measurement data.......................22
Clinical performance data ......................................24
Lifeme...................................................................25
Technical data.........................................................25
Symbols ..................................................................26
Electromagnec declaraon...................................28
Geng support ......................................................31