IME-DC iDia User manual

TABLE OF CONTENTS
NO CODING
USER GUIDE
BLOOD GLUCOSE MONITORING SYSTEM

TABLE OF CONTENTS
3
TABLE OF CONTENTS
INTRODUCTION���������������������������������������������������������������������������5
DESCRIPTION OF SYSTEM COMPONENTS �������������������������������������7
■User interface
■Light button and data port
■Battery compartment
■Test strip
CONFIGURATION �����������������������������������������������������������������������11
■ Conguration
■Date and time
■Test reminder
SYMBOL DEFINITIONS����������������������������������������������������������������17
■‘Insert test strip’ notication
■Blood sampling notication
■Measurement period
■Test result
■Measurement unit
■‘HI’ notication
■‘LO’ notication
■Memory display
■Test reminder
■Ketone notication
ERROR DEFINITIONS AND RECTIFICATION ���������������������������������22
AVERAGE MEASUREMENT CALCULATION������������������������������������24
CONTROL SOLUTION �����������������������������������������������������������������26
■Using the control solution
■Testing with the control solution

TABLE OF CONTENTS
4 5
INTRODUCTION
Thank you for your trust!
You have chosen the iDia blood glucose monitoring system, which
can be used together with iDia test strips and related accessories
to easily and conveniently measure your blood glucose levels.
The measurement range of the iDia blood glucose monitoring sys-
tem is between 10 –600 mg/dL or 0.6 –33.3 mmol/L.
Please ask your specialist physician which unit of measurement
you should use for your therapy.
In order to obtain an accurate blood glucose measurement, you
must follow certain important guidelines.
Therefore, please read this user guide carefully before using the
device for the rst time.
You will be amazed at how easy it is to take measurements!
TABLE OF CONTENTS
LANCING DEVICE������������������������������������������������������������������������30
BLOOD LANCET �������������������������������������������������������������������������31
USING THE LANCING DEVICE������������������������������������������������������32
ALTERNATIVE BODY AREAS SUITABLE FOR BLOOD SAMPLING ����34
DETERMINATION OF BLOOD GLUCOSE LEVEL�����������������������������37
BLOOD GLUCOSE VALUES FOR ADULTS ��������������������������������������39
SYMPTOMS OF LOW BLOOD SUGAR��������������������������������������������40
SYMPTOMS OF HIGH BLOOD SUGAR�������������������������������������������41
POTENTIAL INFLUENCES ON THE MEASUREMENT READINGS�����42
TECHNICAL SPECIFICATIONS – BLOOD GLUCOSE MONITORING
SYSTEM �������������������������������������������������������������������������������������45
TECHNICAL SPECIFICATIONS – TEST STRIPS ������������������������������46
DATA TRANSFER ������������������������������������������������������������������������47
LIST OF ITEMS | QUALITY STANDARDS����������������������������������������48
CLEANING AND MAINTENANCE ��������������������������������������������������49
BATTERY REPLACEMENT������������������������������������������������������������50
WARRANTY ��������������������������������������������������������������������������������52
EXPLANATION OF SYMBOLS�������������������������������������������������������53
SAFETY AND DISPOSAL ��������������������������������������������������������������54
■Blood glucose monitoring system
■Used test strips, lancets and alcohol swabs
■Battery

6 7
INTRODUCTION
Before taking your rst measurement, please take note of the
following important information.
■The iDia blood glucose monitoring system is an in vitro diagnos-
tic tool that is suitable for self-testing. It enables people with
diabetes and specialist medical sta to determine blood glucose
values.
■The iDia blood glucose monitoring system is suitable for analys-
ing fresh capillary blood, venous blood, arterial blood or neonatal
blood.
■Only use iDia test strips and iDia control solutions. Using other
test strips can lead to incorrect readings.
■Close the storage container immediately aer removing the test
strip(s).
■Check the expiry dates of the test strips and control solution.
■Use a new lancet for each blood sample.
■Always store the measuring device or test strips according to the
storage requirements, and protect both from moisture, direct sun-
light and other heat sources.
■Keep the measuring device and all accessories away from chil-
dren.
■Clean your iDia blood glucose monitoring system regularly.
■The functioning of the iDia blood glucose monitoring system
can only be guaranteed if it is used correctly and for its intended
purpose.
■Blood glucose monitoring systems must not be used for diabetes
diagnosis.
Test strip slot
Main button
Display
DESCRIPTION OF SYSTEM COMPONENTS
USER INTERFACE

8 9
Data port
Light button
Pressing the light button when the blood glucose monitoring
system is switched on will activate the display's blue background
lighting. This makes it possible to take and read measurements
correctly even in low light conditions.
Battery
Battery
Set button
DESCRIPTION OF SYSTEM COMPONENTS DESCRIPTION OF SYSTEM COMPONENTS
LIGHT BUTTON AND DATA PORT BATTERY COMPARTMENT

10 11
TEST STRIPS CONFIGURATION
Application area
Touch the drop of blood with the appli-
cation area. The blood will be absorbed
automatically.
Measurement contacts
Insert the test strip into the test strip slot,
with the measurement contacts rst.
Test area
This area must be completely lled with
the blood sample.
MEASUREMENT FIELD
NOTE
You can touch the measurement eld of the iDia test strips freely
with clean, dry hands. The test result will not be aected.
Set button
First remove the battery cover and check that the required 3 V lith-
ium batteries (type CR 2032) have been inserted. If this is not the
case, you will need new batteries (see page 50).
Then set the current date and correct time. To do this, press the set
button (using a pen, for example), and then press the main button
(this changes the ashing value on the display).
NOTE
Please be prepared for necessary battery changes by keeping two
spare batteries with you (Type CR 2032).
CONFIGURATIONDESCRIPTION OF SYSTEM COMPONENTS

12 13
DATE AND TIME
NOTE
The gures needing adjustment ash on and o (shown in white in
the illustrations).
1Set button pressed once: The blood glucose meter switches
itself on. ‘Year’ setting
2Set button pressed second time: ‘Month’ setting
3Set button pressed third time: ‘Day’ setting
4Set button pressed fourth time: ‘Hour’ setting
5Set button pressed h time: ‘Minute’ setting
123
45
TEST REMINDER
6–10 Set button pressed sixth to tenth times:
‘Test reminder’ setting: up to ve dierent test reminder times
can be set (see page 14).
11 Set button pressed eleventh time: The blood glucose meter
switches itself o.
Finally, place the battery cover on the device.
NOTE
If you change settings using the set button and main button, you
will need to go through all of the settings until the blood glucose
11
678
910
CONFIGURATION CONFIGURATION

14 15
CONFIGURATION CONFIGURATION
meter switches itself o (aer pressing the set button for the last
time).
Your changes will only be saved aer this step.
Your iDia blood glucose monitoring system can issue an alarm tone
ve times a day to remind you to check your blood glucose level.
Aer pressing the set button six times (see page 13) you can set ve
dierent times for test reminders.
The main button can be used to turn the test reminder function
on (12) or o (13).
1312
When the test reminder function is activated, you will be prompted
to enter the rst of your preferred times. Press the set button and
then use the main button to set the desired hour (14) and minute (15),
and conrm by pressing the set button again.
Aer setting the rst test reminder, it is possible to set four further
test reminders.
NOTE
You will be reminded to take your measurement by an alarm tone.
To complete the process, press the main button for 1 second.
1514

16 17
‘INSERT TEST STRIP’ NOTIFICATION
16 Aer the iDia blood glucose monitoring system has been
switched on with the main button, the test strip symbol will
appear in the display. You should now insert the test strip into
the device’s test strip slot. Correct insertion of the test strip will
be conrmed by an audio signal.
BLOOD SAMPLING NOTIFICATION
17 Once the test strip has been inserted into the test strip slot, the
blood intake symbol appears in the display. You now have three
minutes to perform a blood glucose measurement. If no blood
sampling takes place within this time, the iDia blood glucose
meter will switch itself o automatically.
SYMBOL DEFINITIONS SYMBOL DEFINITIONS
16 17 18
MEASUREMENT PERIOD
18 Measurement begins once the test area of the test strip is
suiciently lled with blood, and lasts only 7 seconds. During
the measurement, an hourglass animation is visible on the
display.

18 19
TEST RESULT
7 seconds aer the start of the measurement, the blood glucose
value will be displayed and automatically saved, together with the
date and time (19 and 20).
MEASUREMENT UNIT
This will be displayed together with the measurement reading
(21 and 22).
19 20
21 22
‘HI’ NOTIFICATION
This will appear if the measured blood glucose value exceeds
600 mg/dL or 33.3 mmol/L (23 and 24).
‘LO’ NOTIFICATION
This will appear if the measured blood glucose value is below
10 mg/dL or 0.6 mmol/L (25 and 26).
NOTE
If you receive a ‘HI‘ or ‘LO‘ notication, repeat the measurement
procedure. If the notication appears again, perform a measure-
ment using the control solution (see page 27 onwards) or contact
your specialist physician.
23 24
25 26
SYMBOL DEFINITIONS SYMBOL DEFINITIONS

20 21
SYMBOL DEFINITIONS
MEMORY DISPLAY
The iDia blood glucose meter can save up to 900 blood glucose
measurements, along with the date and time of each. Blood glu-
cose measurements and control solution measurements are stored
separately. If the user exceeds the memory capacity, each addi-
tional measurement will automatically overwrite the oldest saved
blood glucose value.
Aer switching on the blood glucose monitoring system and then
pressing the main button, the last blood glucose meter taken will
be displayed with its corresponding date and time (27 and 28). By
pressing the main button again, other saved measurement results
can be recalled.
27 28
SYMBOL DEFINITIONS
TEST REMINDER
29 If the test reminder function is activated, this will be indicated by
an alarm symbol on the display when the blood glucose moni-
toring system is switched on (see also page 13).
KETONE NOTIFICATION
30 If the measured blood glucose value exceeds 300 mg/dL or
16.7 mmol/L, the ketone notication will be automatically dis-
played, which warns you of a potential ketoacidosis.
For more detailed information about ketoacidosis, please consult
with your specialist physician.
29 30
22 23
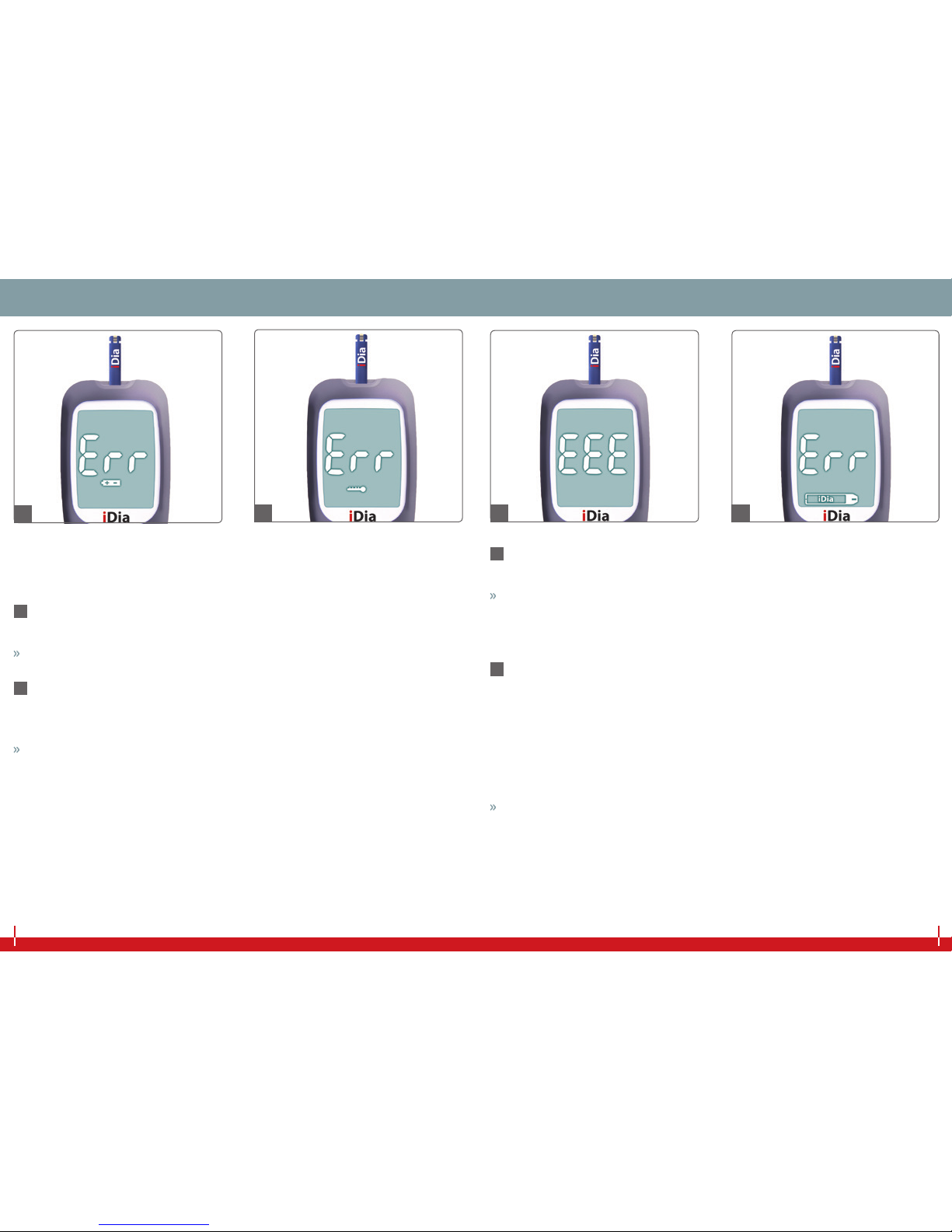
ERROR DEFINITIONS AND RECTIFICATIONERROR DEFINITIONS AND RECTIFICATION
The following errors will be shown on the display with an ‘Err‘ or
‘EEE‘ notication:
31 Err + battery symbol
■Not enough battery power
Change the battery (see page 50 onwards).
32 Err + thermometer symbol
■The ambient temperature is outside the acceptable temperature
range.
The operating temperature must be between +10 °C and +40 °C.
34
31 3332
33 EEE
■Electronic error
In this case, please contact IME-DC customer service.
Service hotline: +49 9281 | 85 01 6-0
34 Err + test strip symbol
■Test strip malfunction
■Used test strip
■Not enough blood absorbed
■Not enough control solution absorbed
■The blood was absorbed before the blood sample symbol ap-
peared in the display.
Repeat the measurement with a new test strip.
24 25
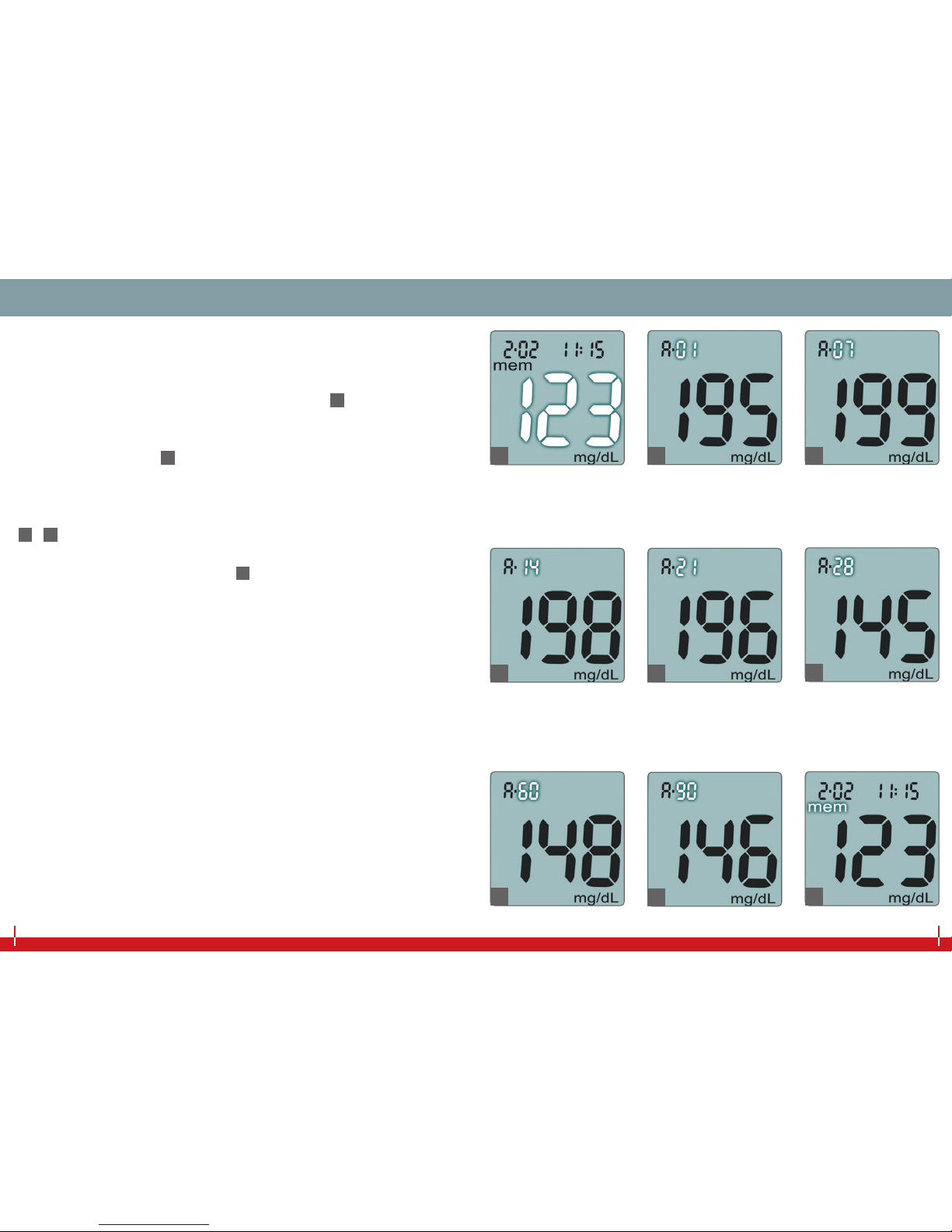
AVERAGE CALCULATION OF MEASUREMENTSAVERAGE CALCULATION OF MEASUREMENTS
Your iDia blood glucose meter allows you to call up and display the
average values of the last 24 hours and the past 7, 14, 21, 28, 60 and
90 days.
To do this, please activate the memory display (35) (see page 20).
Simultaneously press the main button and light button and hold
them for approximately 2 seconds. The average value of the last
24 hours is displayed (36).
Each time you press the main button again, the average values for
other periods (the past 7, 14, 21, 28, 60 or 90 days) will be displayed
(37 - 42).
To return to the memory display (43), press the main button and
light button simultaneously and hold them for 2 seconds again.
35 36 37
38 39 40
41 42 43

26 27
CONTROL SOLUTION CONTROL SOLUTION
USING THE CONTROL SOLUTION
Test measurement with the iDia control solution is used to check
that the blood glucose monitoring system is functioning correctly.
This procedure is recommended in the following cases:
■For teaching and training purposes
■If the blood glucose meter or the blood glucose test strips have
been stored at an inappropriate temperature
■Following improper handling of the blood glucose meter
■In the event of questionable blood glucose readings
NOTES
■The control solution must not be used aer the expiry date.
■When the control solution bottle is rst opened, the date must be
written on the label.
■The shelf life of the control solution is 90 days aer it is rst
opened.
■Always take care that the control solution is used correctly.
TESTING WITH THE CONTROL SOLUTION
Insert an iDia test strip into the test strip slot. The blood glucose
monitoring system will switch itself on automatically.
44 The display will now prompt you to take a blood sample.
45 Press the main button and hold it for approximately 2 seconds
until the control solution symbol appears in the display.
You are now in the designated control solution measurement
mode. In this mode, measurement results of the control solution
are saved separately.
This prevents the average values of your personal blood glucose
measurements being falsied by the control solution measure-
ments.
44 45

28 29
Next, rotate the control solution bottle 3 – 4 times.
Please don’t shake it!
46 Aer opening the bottle, put a drop of control solution on a
clean, dry surface (for example, the lid of the test strip contain-
er), at a distance of approximately 2 cm. Then close the bottle.
Use a new drop of control solution for each measurement.
47 Now place the application area of the test strip on the drop of
control solution, until the test area is completely lled. An audio
signal will conrm the start of the measurement.
46 47
CONTROL SOLUTION CONTROL SOLUTION
Aer 7 seconds, the measurement reading will be shown together
with the date, time, measurement unit and bottle symbol (48 and 49).
The measurement reading should fall within the appropriate target
range (see test strip container label).
The test result lies outside the control range
If an error message is displayed during measurement, or if the
measured value is outside the target range (as shown on the test
strip container), repeat the measurement. If an error message ap-
pears again or if the measurement is again outside the target range,
please contact IME-DC customer service.
Service hotline: +49 9281 | 85 01 6-0
48 49

TABLE OF CONTENTSTABLE OF CONTENTS
30 31
LANCING DEVICE BLOOD LANCET
Protective cap
Spring clip Lancet body
Slot
Activation button
Lancet
Lancing device cap
Lancing device regulator
(can be adjusted individually)

32 33
USING THE LANCING DEVICE
49 Unscrew the lancing device cap.
50 Insert the lancet into the slot.
51 Twist o the lancet protection cap.
52 Screw the lancing device cap back on.
53 Set your personal puncture depth (minimum 1, maximum 5).
54 Load the lancing device by pulling the spring clip back.
49 57
52 60
50
53
51
54
USING THE LANCING DEVICE
55 Press the tip of the lancing device against your nger and press
the activation button.
56 The blood drop obtained can be used for the measurement
procedure.
57 Unscrew the lancing device cap.
58 Press the lancet into the protective cap.
59 Slide the spring clip forward sharply, to eject the lancet.
60 Screw the lancing device cap back on.
55 56
58 59

TABLE OF CONTENTS
34 35
ALTERNATIVE BODY AREAS SUITABLE FOR BLOOD SAMPLING
Capillary blood for blood glucose measurements can be obtained
not only from the ngertips, but also from other areas of the body
(palms, forearms, upper arm, or calves). This is known as Alterna-
tive Site Testing (AST).
Talk to your specialist physician rst if you would like to use blood
from alternative sites for your blood glucose measurements.
NOTE
Measurement errors can lead to incorrect medical treatment re-
commendations and therefore to serious health problems. Read
these instructions in full before using blood from alternative sites
for your blood glucose measurements.
Restrictions
The following restrictions must be taken into account before taking
blood measurements from alternative sites. Capillary blood in the
ngertips reacts more quickly to changes in blood glucose levels
than that in alternative areas of the body. For this reason, blood
glucose values from alternative sites may dier from those mea-
sured from the ngertips.
Do NOT use blood from alternative sites of your body:
■If your last meal was less than two hours ago, as blood glucose
values change rapidly during this period
■ Aer sports activities
■If you are ill with an acute fever or if you are bedridden
■If you suspect that you have a very low blood glucose level (low
blood sugar)
ALTERNATIVE BODY AREAS SUITABLE FOR BLOOD SAMPLING
■If you know that sometimes you don't recognise having low blood
sugar
■ During the period when the eectiveness of normal insulin or
rapid-acting insulin analogues is at its maximum
■ If your last injection of a rapid-acting insulin analogue was less
than two hours ago
NOTE
If the measurement obtained using blood from an alternative site
does not correspond to your current state of health, you should
perform a measurement using blood from the ngertips.
If you wish to obtain blood samples from alternative sites, you can
order a special cap for the lancing device in your iDia blood glucose
monitoring system set from IME-DC customer service.
Unscrew the lancing device cap. Aer inserting the lancet, screw on
the AST cap (61).
61

TABLE OF CONTENTS
36 37
DETERMINATION OF BLOOD GLUCOSE LEVEL
62 Only use iDia test strips for the iDia blood glucose meter.
63 Wash your hands with warm water and dry them well before
taking a measurement.
64 Insert the test strip into the test strip slot on the iDia blood
glucose meter. The blood glucose meter system will switch itself
on automatically.
If the temperature is within the permitted range, the blood
glucose meter will prompt you to touch the blood drop with the
application area (see page 10 onwards).
Now use the lancing device to obtain a blood drop (see 49 –60
from
page 32 onwards).
62 63 64
ALTERNATIVE BODY AREAS SUITABLE FOR BLOOD SAMPLING
Preferred sampling sites:
Palm of the hand below the little
nger
Palm of the hand below the thumb
Inner side of the forearm
Outer side of the forearm
Upper arms
Thighs
Calves
Balls of the feet for newborns

38 39
DETERMINATION OF BLOOD GLUCOSE LEVEL
Position the application area of the iDia test strip to the blood drop
(65). The blood will be absorbed automatically. An audio signal will
conrm that measurement has started.
Aer 7 seconds, the measurement reading will be displayed and
saved automatically, together with the date, time and unit of
measurement (66 and 67).
66 67
65
BLOOD GLUCOSE VALUES FOR ADULTS
Blood glucose values for adults1
(Reference sample type: venous plasma)
without diabetes with diabetes
Empty stomach ≤ 100 mg/dL
≤ 5.6 mmol/L
≥ 126 mg/dL
≤ 7.0 mmol/L
2 hours aer a meal ≤ 140 mg/dL
≤ 7.8 mmol/L
≥ 200 mg/dL
≤ 11.1 mmol/L
1R. Landgraf et al., 2013: Praxisempfehlungen DDG/DGIM [Recommended prac-
tice – German Diabetes Association/German Association for Internal Medicine];
Diabetologie und Stowechsel [Diabetology and metabolism]; Thieme Verlag
Other manuals for iDia
1
This manual suits for next models
1
Table of contents
Other IME-DC Blood Glucose Meter manuals
Popular Blood Glucose Meter manuals by other brands

Abbott
Abbott FreeStyle Flash Blood Glucose Monitor owner's booklet
Keto-Mojo
Keto-Mojo TD-4279 owner's manual

Advocate
Advocate Redi-Code+ BMB-EA001S user manual

Bayer HealthCare
Bayer HealthCare CONTOUR user guide

Bayer HealthCare
Bayer HealthCare Ascensia Contour Quick reference guide

hivox
hivox Dreamate DM-800 instruction manual