Innovo INV-942R iSoothe User manual
User Manual
Clinically
Proven
By
By
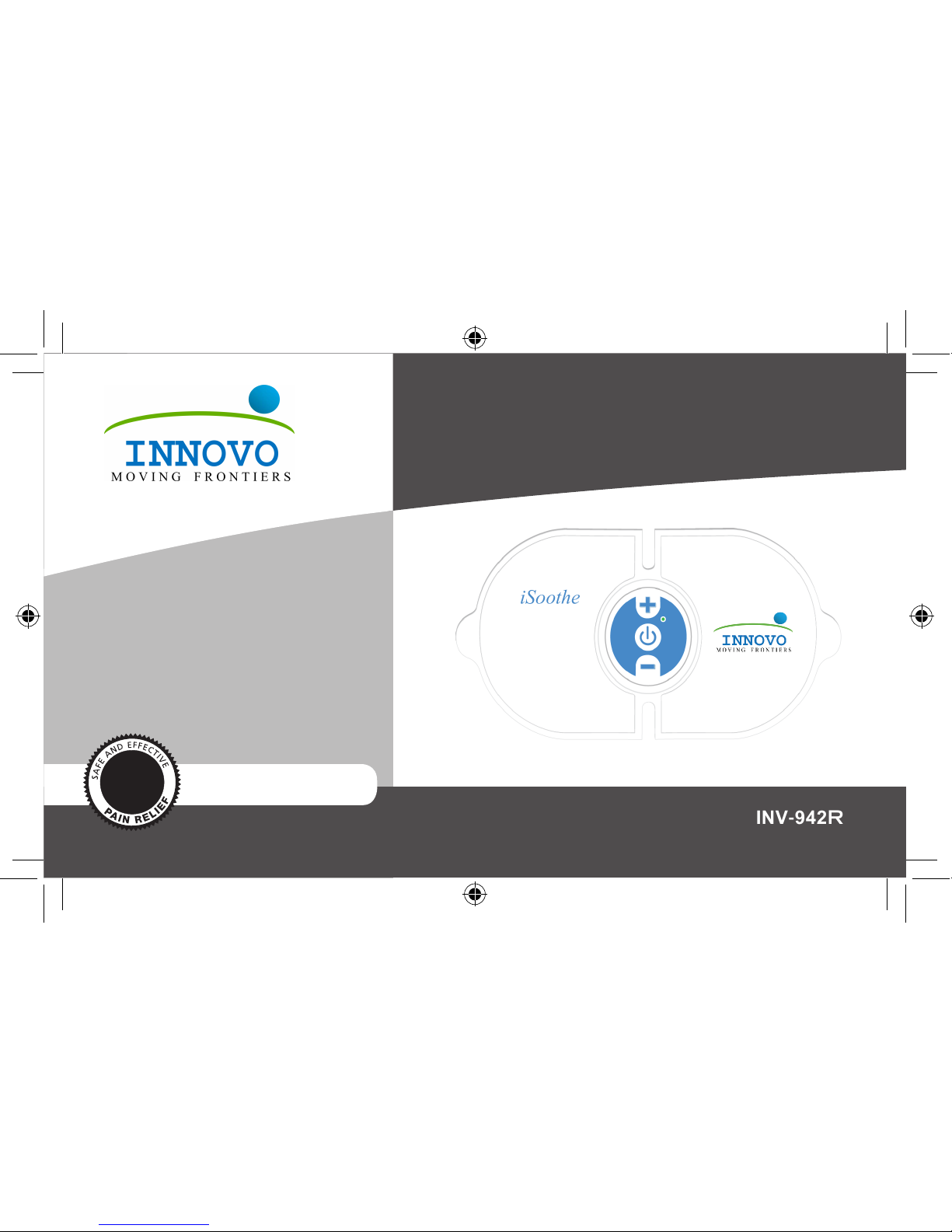
iSoothe™
WIRELESS RECHARGEABLE TENS
INV-942R
•Rechargeable Battery
•20 Minute Treatment Timer
•5 Preset Modes
•15 Intensity Levels
•Auto Shut-Off
3
This manual is applicable to the iSoothe Wireless Rechargeable TENS INV-942R.
This instruction manual is published by Innovo Groups.
Innovo Groups reserves the right to update this manual at any time without prior notice.
Amendments may however be published in new editions of this manual.
All Rights Reserved. Rev. V1.0 © 2016, 160101
Conformity to safety standards
Innovo Groups declares that the device complies with the following normative documents: ISO13485
CMDCAS, ISO9001 CERT, ISO13485 UKAS
TABLE OF CONTENTS
Introduction .............................................................................................................................. 4
Important safety precautions and warnings ............................................................................... 6
Package contents................................................................................................................... 13
Know your device ................................................................................................................... 14
Battery information ................................................................................................................. 15
Treatment information ............................................................................................................. 15
Gel pad positioning ................................................................................................................ 21
Maintenance and cautions ..................................................................................................... 22
Storage................................................................................................................................... 22
Technical specifications........................................................................................................... 23
Program ................................................................................................................................. 24
Disposal.................................................................................................................................. 25
Troubleshooting ...................................................................................................................... 26
Important information regarding electromagnetic compatibility (EMC)....................................... 27
Explanation of symbols ........................................................................................................... 34
Warranty................................................................................................................................. 35
4 5
How does TENS work?
Scientific theory suggests that electrical
stimulation therapy may work in several ways:
nThe gentle electrical pulses block the "pain
message" from the nerves from reaching the
brain.
nThe gentle electrical pulses induce an increase
in the productions of endorphins, the body’s
natural pain killer.
INTRODUCTION
Thank you for purchasing the Innovo iSoothe™
Wireless Rechargeable TENS (Model INV-942R)
for your pain relief solution.
Please read the manual carefully before using the
device.Keep this instruction manual in a secure
location with the device forfuturereference.
The pain reliever INV-942R is a TENS stimulator.
What is TENS?
TENS stands for Transcutaneous Electrical Nerve
Stimulation. It is a noninvasive, drug-free method
of managing pain. TENS uses tiny electrical
impulses sent through the skin to the nerves to
modify pain perception. TENS does not cure any
physiological problem. It only helps to manage
the pain. Please note that itmight not work for all
patients,
What conditions can TENS help relieve?
TENS provides pain relief for a number of
different pain conditions associated with exercise,
daily work and household activities. This product
is designed for the temporary relief of muscle,
joint and bone pain in the:
nNeck nWaist
nShoulder nUpper Extremities (arms)
nBack nLower Extremities (leg)
The pain reliever should be applied to
normal, healthy, clean and dry skin of
adult patients.
What can I treat?
The TENS device can be used to manage many
different types of pain. Please refer to the
diagrams on page 21 for the ideal locations to
place the gel pads for the treatment of the most
common forms of pain. For other areas of pain,
place the gel pads on either side of the pain area.
PLEASE NOTE: Never place the gel pads on the
head, throat, face, heart, chest area, eyes, oral
cavity, sexual organs or over the spine or bony
areas.
How long can Iuse the Wireless TENS unit?
We recommend that you use the TENS unitfor an
average of 20 minutes aday for each area of
treatment. For longer duration, please seek
medical advice. If you exceed the recommended
time,please check your skin wherethe gel pads
have been placed toensure that your skin does
notbecome sore.
PLEASE NOTE: The gel pads are designed for
temporary use. It should last for approximately 10
days when used for 20 minutes a day if stored &
used as directed.
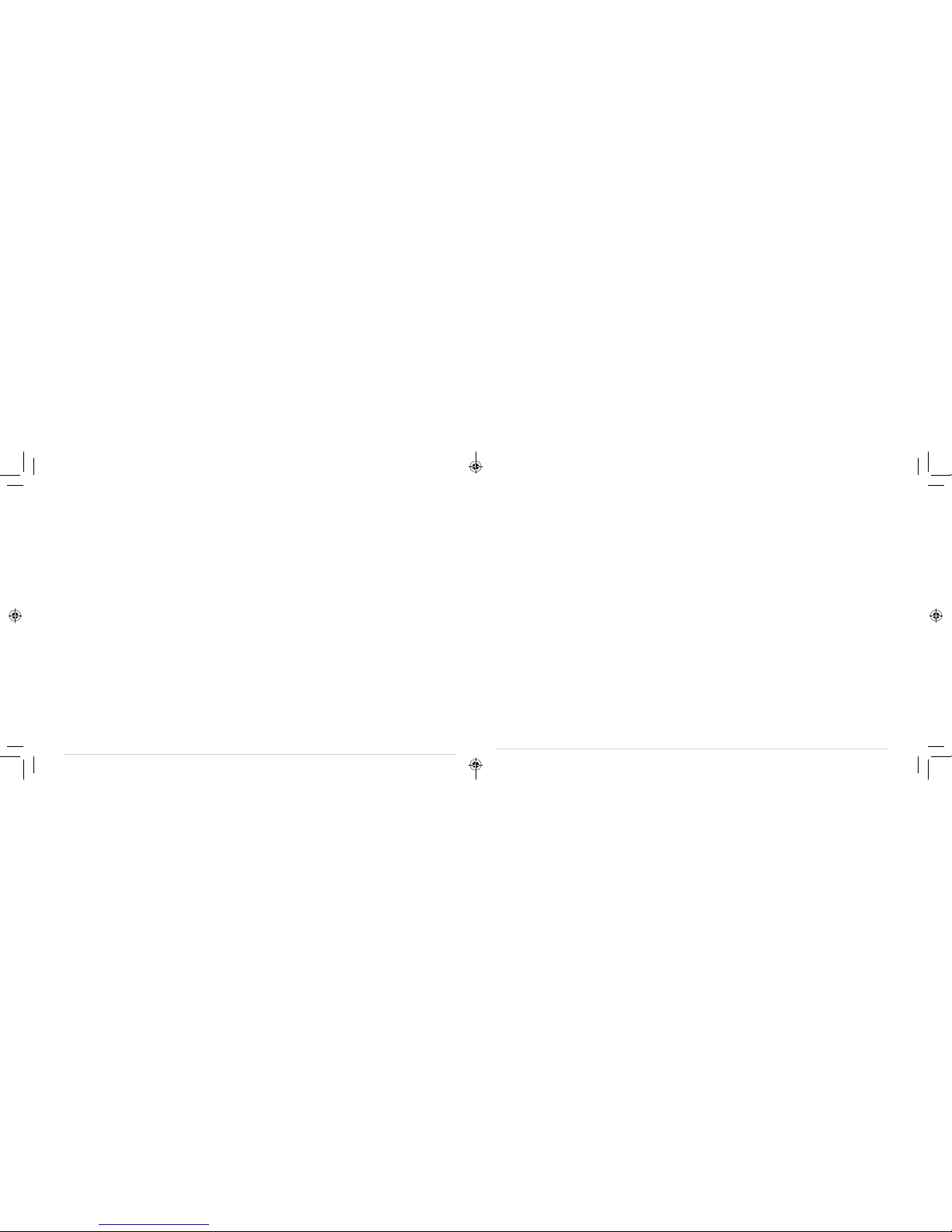
Package Contents:
n1 x TENS Device n2 x Gel pads
n1 x Wall Charger
n1 x Plastic Case
n1 x User manual
n1 x USB Cable
n1 x Carry Bag
6 7
SAFETY SYMBOLS USED IN THIS MANUAL
Indicates a potentially hazardous situation
which, if not avoided, could result in death
or serious injury.
Indicates a potentially hazardous situation
which, if not avoided, could result in
serious injury and equipment damage.
Indicates a potentially hazardous situation
which, if not avoided, may result in minor
or moderate injury to the user, or damage
to the device or other property.
IMPORTANT SAFETY PRECAUTIONS AND WARNINGS
It is important that you read all the warningsand precautions included in this manual because
they are intended to keep you safe, prevent injury and avoid a situation that could damage the
device.
DANGER
This stimulator must not be used in
combination with the following medical
devices:
nInternally transplanted electronic medical
devices, such as pacemakers.
nElectronic life-support equipment, such as
respirators.
nElectronic medical devices attached to the body,
such as electrocardiographs.
Using this stimulator with other electronic medical
devices may cause erroneous operation of those
devices.
WARNING
Consult with your physician before using
this device. The device may cause lethal
rhythm disturbances in certain susceptible
individuals.
DO NOT USE THIS DEVICE UNDER
THESE CONDITIONS:
nIf you have a cardiac pacemaker, implanted
defibrillator, or other implanted metallic or
electronic device. Such use could cause electric
shock, burns, electrical interference, or death.
nTogether with a life-supporting medical
electronic device such as an artificial heart, lung
or respirator.
nIn the presence of electronic monitoring
equipment (e.g., cardiac monitors, ECG alarms),
which may not operate properly when the
electrical stimulation device is in use.
nOn open wounds or rashes, over swollen, red,
infected, inflamed areas, or skin eruptions (e.g.,
phlebitis, thrombophlebitis, varicose veins); or
on top of, or in proximity to, cancerous lesions.
nOver areas of skin that lack normal sensation.
nOn the opposite sides of your head since the
effects of stimulation of the brain are unknown.
DO NOT USE ON THESE INDIVIDUALS:
nPregnant women, because the safety of
electrical stimulation during pregnancy has not
been established.
nChildren or infants, because the device has not
been evaluated for pediatric use.
nPeople incapable of expressing their thoughts
or intentions.
8 9
DO NOT USE THIS DEVICE DURING
THESE ACTIVITIES:
nBathing or showering;
nSleeping;
nDriving, operating machinery or any activity in
which electrical stimulation can put you at risk
for injury.
PAIN MANAGEMENT WARNINGS
nIf you have prior medical or physical treatment
for your pain, consult with your physician before
using this device.
nIf your pain does not improve, becomes
seriously chronic or severe, or continues for
more than five days, stop using the device and
consult with your physician.
nPain is an indicator that you might be ill.
Therefore, ifyou are suffering from any
serious illness, consult your physician to
confirm that you can use the TENS unit.
WARNINGS AND PRECAUTIONS
REGARDING THE GEL PADS
nApply pads only to normal, healthy,clean and
dry skin (of adult patients) because it may
otherwise disrupt the healing process.
nIf you experience any skin irritation or redness
after a session, discontinue use immediately.
NEVER APPLY THE PADS TO:
n The head or any area of the face.
nThe neck or any area of the throat because
this can cause severe muscle spasms
resulting in the closure of airway, difficulty in
breathing, or adverse effects on heart rhythm
or blood pressure.
nBoth sides of the thorax simultaneously (lateral
or front and back), or across your chest
because the introduction of electrical current
may cause rhythm disturbances, which could
be lethal.
CAUTION
WARNINGS AND CAUTIONS REGARDING
THE GEL PAD
nDo notbend or fold because it may prevent the
pad from functioning properly.Place the pad
onto the plastic film and store in the plastic
case when not in use.
nDo not apply ointment or any solvent to the
pads or your skin because it will prevent the pad
from functioning properly.
nThe pad is already pre-gelled and will adhere to
your skin.
nTo avoid damage to the adhesive surface of
the pad, put the pad only on clean and dry
skin or on the p lastic film provided.
nAlways place clean pad in accordance with
the illustrations provided (Refer to page 2 1
for electrode placement).
nThe spine or backbone.
nAny metal object, such as a belt buckle,
necklace or other jewelry made from
metal.
10 11
nDo not share pad with another person. This
may cause skin irritation or infection. Pad is
intended for use by one person only.
nDo not place or relocate the pad while the
device is on.
nAlways turn the power off before removing or
changing the pad location.
nDo not leave pad attached to the skin after
treatment.
CAUTION WHILE USING THE TENS UNIT
nIf the TENS unit is not functioning properly or
you feel discomfort, stop using the
device immediately.
nDo not use for any other purpose except as
described in this manual.
nDo not use the TENS device while wearing
electronic devices such as watches as this may
damage the device.
nDispose the device and components
according to applicable legal regulations.
Unlawful disposal may cause environmental
pollution.
nThe size, shape and type of pads may affect the
safety and effectiveness of electrical
stimulation. Use only iSoothe™electrodes
(iSoothe™Pain Relief Pad Supply Kit – E-946R)
designed specifically for the INV-942R Wireless
TENS device.
GENERAL PRECAUTIONS
nThe long-term effects of electrical stimulation are
unknown.
nApply stimulation to only normal, intact, clean,
dry, and healthy skin.
nTENS is not effective in treating the original
source or cause of the pain, including
headache.
nTENS is not a substitute for pain medications
and other pain management therapies.
nTENS devices do not cure diseases or injuries.
nTENS is a symptomatic treatment and, as
nEffectiveness is highly individual dependent
nYou may experience skin irritation or
hypersensitivity due to the electrical stimulation
or electrical conductive medium (gel) on the
electrodes.
nIf you are at risk or diagnosed with a heart
disease, you should follow precautions
recommended by your physician.
nIf you are at risk or diagnosed with epilepsy,
you should follow precautions recommended by
your physician.
nUse caution if you have a tendency to bleed
internally, such as following an injury or fracture.
nConsult with your physician prior to using
the device after a recent surgical procedure,
because stimulation may disrupt the healing
process.
nThis stimulation should notbe applied on
menstruating or pregnant uterus.
nThis stimulation should not be applied to
areas of skin that lack normal sensation.
such, suppresses the sensation of pain
that would otherwise serve as a warning
mechanism.
12 13
nKeep unit away from young children.
The unit contains small pieces that may
be swallowed and may cause choking.
Contact your physician immediately if
ingested.
nUse this device only with iSoothe™electrodes.
POSSIBLE ADVERSE REACTIONS
nDo not use the device to treat one region for an
extended periods of time (more than 20
minutes a session, up to 2 times/day). This may
cause exhaustion and soreness to the muscles
in the treated region.
nYou may experience skin irritation and burns
beneath the stimulation electrodes.
nYou should stop using the device and consult
with your physician if you experience adverse
reactions from using the device.
PACKAGE CONTENTS
PACKAGE CONTENTS
Protective Case
Draw String Carrying Bag
Instruction manual
User Manual
Clinically
Proven
By
By
iSoothe™
Wireless Rechargeable TENS
INV-942R
• Rechargeable Battery
• 20 Minute TreatmentTimer
• 5 Preset Modes
• 15 Intensity Levels
• Auto Shut-Off
Power Adapter & USB Cable
Two (2) Electrode Pads
E-946R
INV-942R unit
By
By
iSoothe™
Wireless Rechargeable TENS
iSoothe
14 15
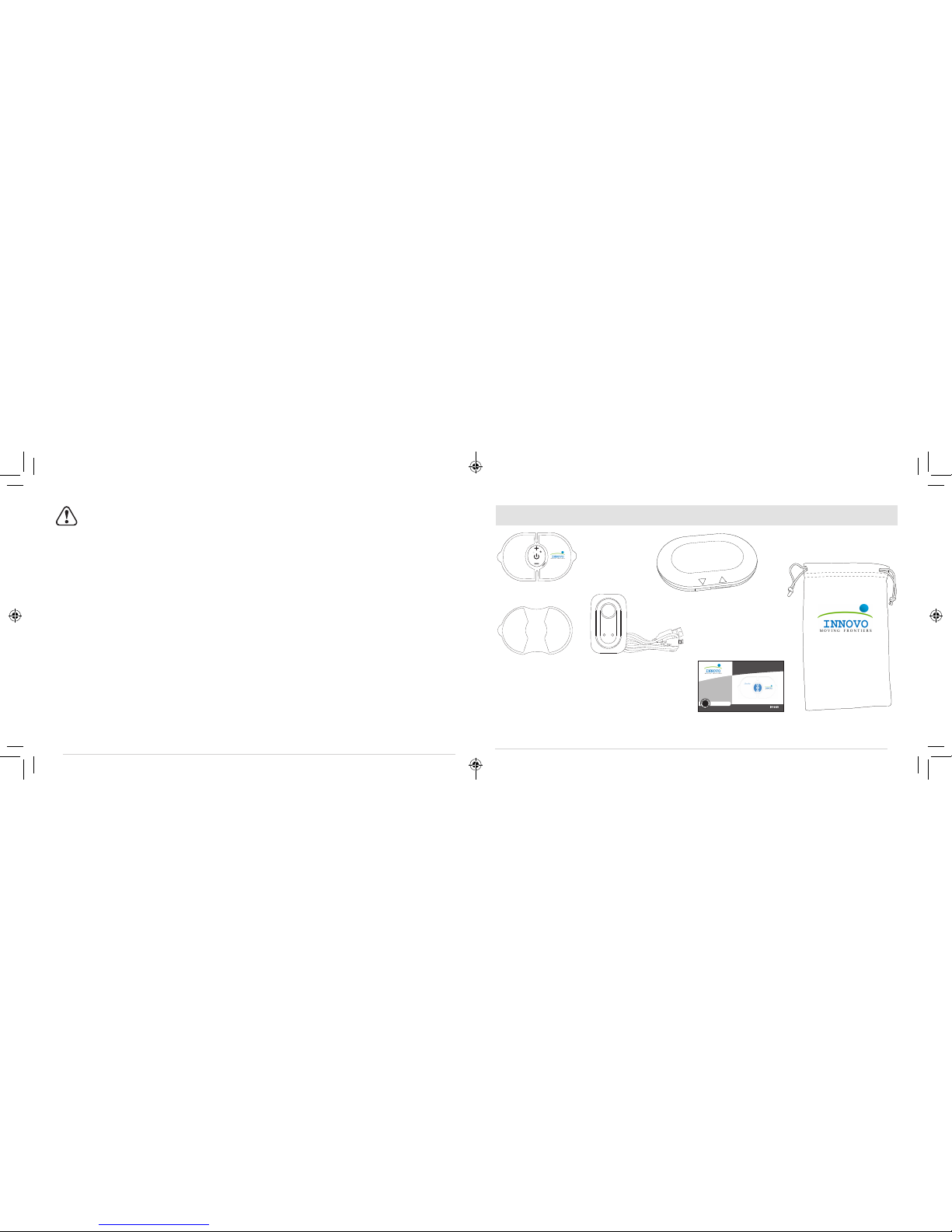
KNOW YOUR DEVICE
Instructions For Use.
1. Battery comes precharged and is ready for use.
2. Battery should be charged by either connecting to
a computer via USB orwall charger provided. A
red light on the charger means that the device
requires charging. Green light means the device is
fully charged. When red and green light appears
simultaneously, this means that the device is not
connected to the charger. If you are charging the
device via USB only,please let the device charge
for at least 2 hours (as there is no indicator light on
the device to show a complete charge). Once fully
charged, the battery should last for at least 8
hours (depending on intensity level used).
Standard For Battery Recharge
1. If you feel the stimulation is weak, the LED is
dim or the device cannot power on, the battery
may need to be recharged.
BATTERY INFORMATION
STEP 1
Instructions For Use
Before use, it is recommended to charge the
iSoothe™ device with the enclosed USB
Cable/wall charger provided.
STEP 2
Cleaning ofskin
Clip excess hair from the treatment area.
Wash area with soap and water, and dry
completely.
TREATMENT INFORMATION
Intensity Decrease and
Mode Change
Intensity Increase and
Mode Change
LED Light Indicator
Sound Indicator
USB Port
On/Off
Note: Product image for illustration purposes only. Actual product may vary.
16
17
STEP 3
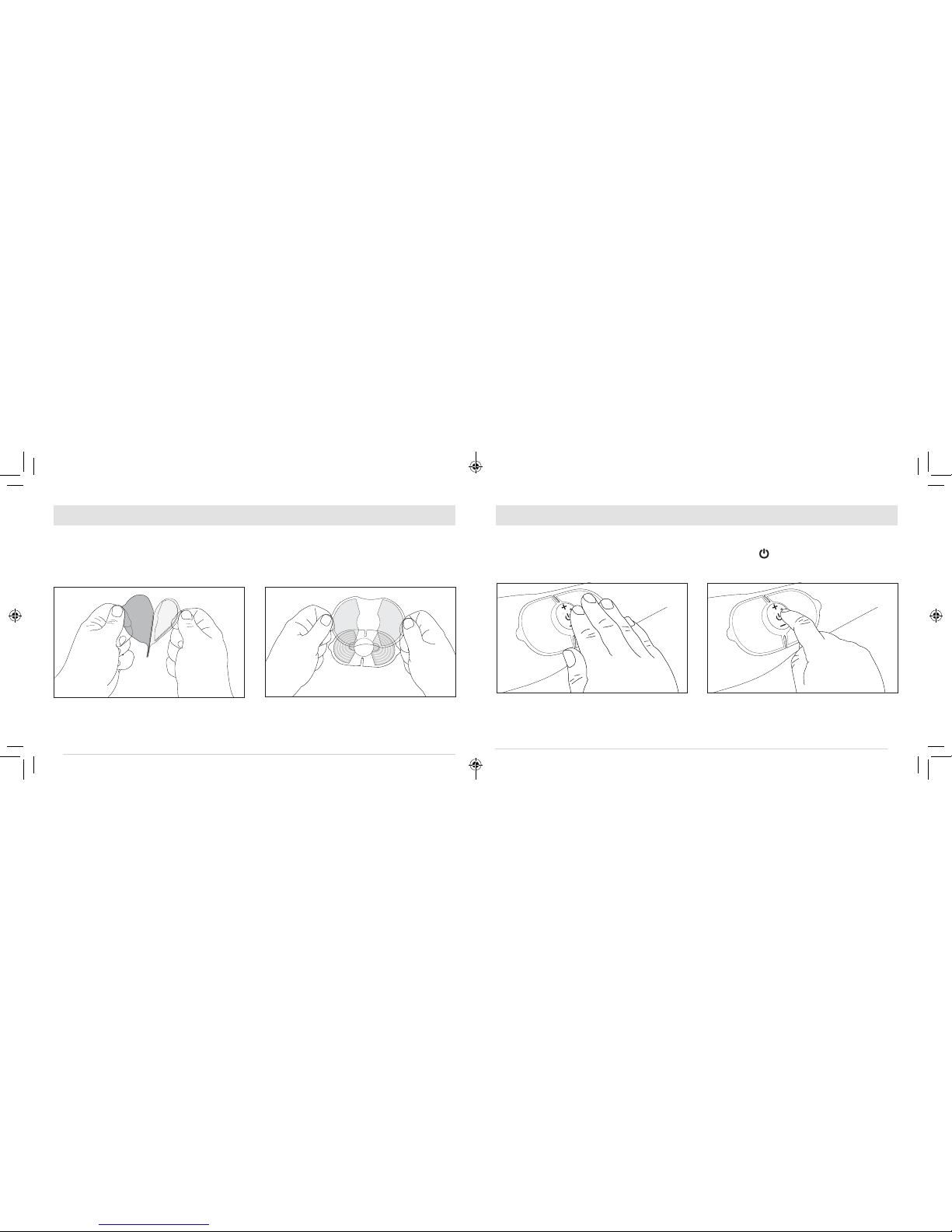
Preparation of the TENS unit
nTear open the sealed electrode pad package.
Peel off the blue plastic film,starting from one
side of the enclosed gel electrode pad.
nTurn the main unit over and line up the sticky
pads with the silver pads on the bottom of
the main unit &press firmly to ensure the
adhesive is securely attached to the device.
Note: Keep the protective transparent film. You
will need to put it on the pad when the pad
is not in use.
TREATMENT INFORMATION (cont.) TREATMENT INFORMATION (cont.)
TM
STEP 4
Placing the TENS unit
nPlace the pad on the treatment area (see page
21 for placement suggestions). Press
down firmly to ensure full contact with skin.
NOTE: GEL PADS ARE REPLACEABLE.
iSoothe™Pain Relief Pad Supply
K it Item #E-946R
STEP 5
Power On
nPress the " "button to turn the indicator
on. The LED will light up and you will hear a
beep for one (1) second.
nWhen turned on, the device will work at Pulse
Mode 1 by default.

18 19
the device.
nTo change the mode, press the “+”button for
3 seconds to select the desired pulse mode
(Mode 1 – 5). See chart to the right.
nBy pressing the “–” button for 3 seconds you
can also select the desired pulse mode in
the opposite order.
nWhen the mode changes, the indicator light
will flash twice and the sound will beep twice.
nSee Step 7 for changing the intensity levels.
STEP 7
Changing Intensity Levels
nAfter mode selection, press the “+” or “–” button
to increase or decrease the pulse intensity as desired.
nEach time the intensity is increased or decreased
by pressing the “+”or “–” button,the LED will
flash once with a beep. There are 15 intensity
levels in total.
MODE TYPE
1Scraping
2Massage
3Tapping
4Acupuncture
5Combo
NOTE:
See page 24 for mode details.
TREATMENT INFORMATION (cont.)
STEP 6
Changing Modes
nMode 1 is the default setting when you turn on
TREATMENT INFORMATION (cont.)
STEP 8
Timer
nOnce you have found the desired intensity/
Mode, the timer will begin.
nThe timer counts down from 20 minutes.
nWhen the timer is up the unit will turn off
automatically. The unit can also be turned
off by pressing . The light and sound
will blink and beep 3 times to indicate that
the device is powering down.
NOTE:
To conserve battery and prevent unexpected
shock, the device will automatically power off
when it is not inuse on any treatment area.
This is indicated by a single 5 seconds beep
with light, followed by 5 beeps & flashes.
20 21
TREATMENT INFORMATION (cont.)
PLEASE NOTE:
nDo not move the gel pad to another part of your
body without turning off the power.
nKeep the gel pads clean and do not expose to
heat or direct sunlight.
nIf the gel pads do not adhere to your body or
are dirty, replace with a new replacement
gel pad (item# E-946R).
nDo notclean the pad or adhesive gels with any
chemical.
nPlace the gel pads on clean skin only.Donot
place on cuts or damaged skin.
nThe iSoothe™is for single person use.
nPlace the blue plastic film on the gel pad when
not in use.
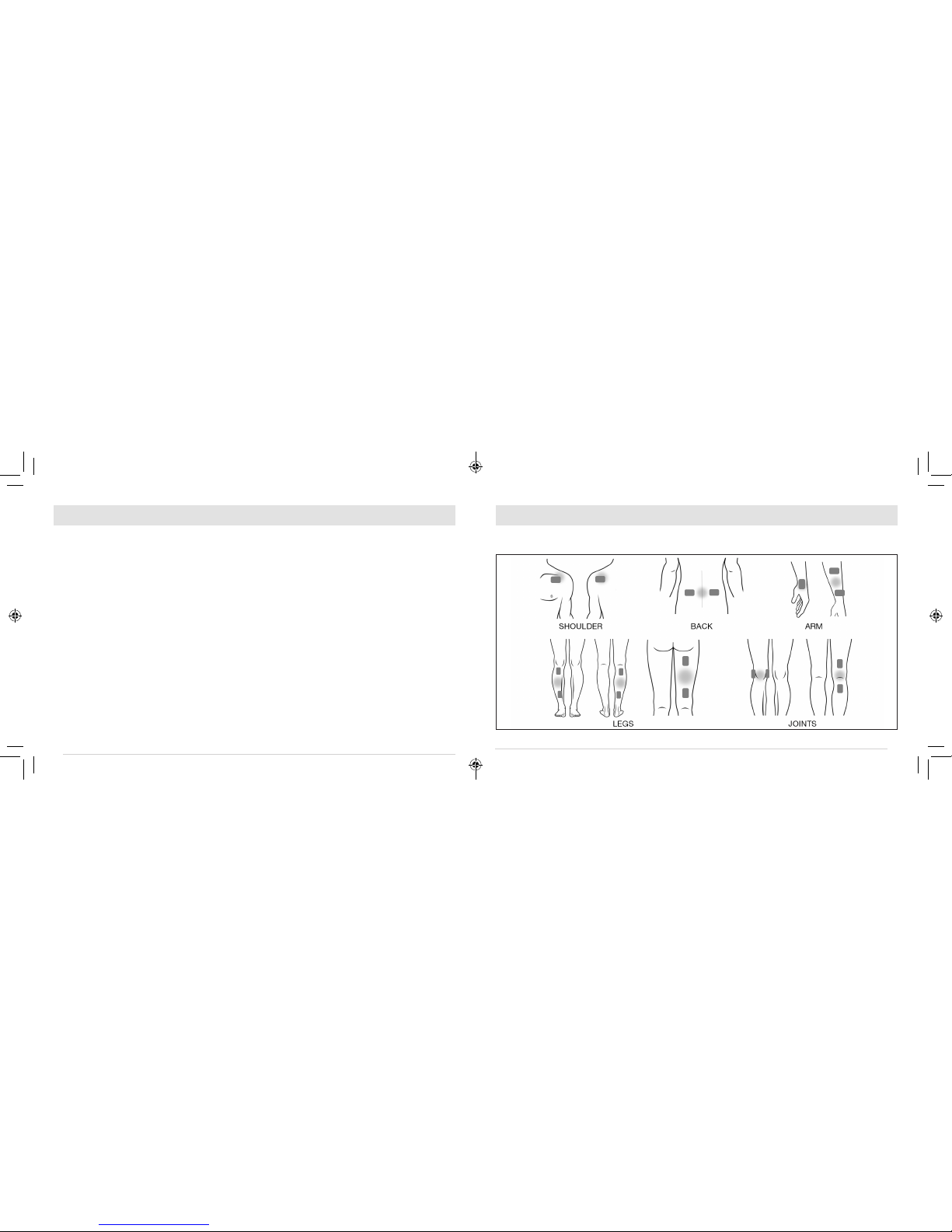
Front of
Shoulder Back of
Shoulder
GEL PAD POSITIONING
NOTE: The illustrations below show 2 suggested treatment sites per body part. However, only one device
should be used at a time.

22 23
MAINTENANCE AND CAUTIONS
nDo notimmerse the iSoothe™INV-942R
in water or any liquid. Do not drop the
device or throw it from a height.
nAfter use, remove the gel pads from the skin
and place on the protective transparent film.
nAlways use the protective film when the gel
pads are not in use.
nDo not use any chemical to clean the device
or the gel pads. If you need to clean the unit,
please wipe with a damp, lint-free cloth.
nDo not let the gel pads dry out or expose them
to direct sunlight.
nKeep the gel pads clean.
STORAGE
nAfter use, disconnect the gel pads. Store
safely and out of the reach of children.
nStore the iSoothe™INV-942R in a cool, dry
place, -14°F–131°F;10% –95%
relative humidity.
nDo notexpose the gel pads todirect sunlight
and protect them against dirt and moisture.
nIf the gel pads no longer adhere to your skin or
or the gel pads are broken, you should replace
with new pads.
nThe iSoothe™INV-942R is intended for single
patient use. Do notshare with others.
TECHNICAL SPECIFICATIONS
Type
Power supply
Wave form
Frequency
Pulse width
Intensity
Mode
Output voltage
Output intensity level
Treatment time
Operating conditions
Storage conditions
Size
Device Weight
iSoothe
™
Wireless Rechargeable TENS
(INV-942R) DC3.7V Rechargeable Battery
Bi-phase square pulse wave
0~ 200Hz
100
μS
15 Levels
5 Modes
20mA
0~15 levels
20 minutes
14°F~104°F (-10°C~40°C); 30%RH~ 85%RH
14°F~131°F (-10°C~55°C); 10%RH~ 95%RH
11.7 (L) x 7.1 (W) x 1.1 (H) cm
0.04 oz.
NOTE: Design and specifications are subject to change without notice.
24 25
Mode Frequency Pulse width Cycle Time Description
135.7Hz 50 – 100
μS
5 sec. ON
1 sec. OFF
The pulse width changes in a cycle, ranging from
50 – 100
μS at a
frequency of 35.7Hz. The cycle time is 6 seconds.
260Hz 50 – 100
μS
2.5 sec. ON
1 sec OFF
The pulse width changes in a cycle, ranging from
50 – 100
μS at a
frequency of 60Hz. The cycle time is 3.5 seconds.
36.6Hz 50 – 100
μS
150 mS
The pulse width changes in a cycle, ranging from
50 – 100
μS at a
frequency of 6.6Hz. The cycle time is 150 milliseconds.
480Hz 50 – 100
μS
7.5 sec. ON
1 sec. OFF
The pulse width changes in a cycle, ranging from
50 – 100
μS at a
frequency of 80Hz. The cycle time is 8.5 seconds.
5N/A N/A N/A Cycles through modes 1 – 4 at 30 second intervals.
PROGRAM
The iSoothe
™
INV-942Runit ispreset with acombination program that delivers three phases of alternating therapy.
They are specified as follows:
DISPOSAL
When the battery is dead and will not hold a charge any more, the unit must be disposed of in a specially-
labeled collection container, at toxic waste collection points or through an electrical retailer. You are under
legal obligation to dispose of batteries correctly.
Please dispose of the device in accordance with appropriate laws.
26 27
TROUBLESHOOTING
PROBLEM POSSIBLE CAUSES POSSIBLE SOLUTION
The unit does not
power on
The battery is exhausted Recharge the battery.
Stimulation is weak
or cannot feel any
stimulation
Gel pads are dried out or dirty. Replace with new gel pads.
Gel pads cannot adhere to skin properly.Clean treatment area of dirt and/or oily
substances (lotion).
Battery has a low charge Recharge the battery.
Stimulation is
uncomfortable
Intensity is too high. Decrease intensity.
Device is not operated according to the manual instructions Please check the manual before use.
The skin becomes
red and/or you feel
a stabbing pain
Using the gel pad on the same site every time. Re-position the gel pad.
The gel pad is not sticking onto the skin properly. Ensure that the gel pad is securely
placed on treatment area.
The gel pad is dirty. Replace with new gel pad.
The surface of the gel pad is scratched. Replace with new gel pad.
If problems persist. Contact your physician.
If the unit does not operate after taking these measures, contact your nearest dealer. IMPORTANT INFORMATION REGARDING ELECTROMAGNETIC COMPATIBILITY (EMC)
With the increased number of electronic devices
such as computers and mobile (cellular) telephones,
medical devices in use may be susceptible to
electromagnetic interference from other devices.
Electromagnetic interference may result in incorrect
operation of the medical device and create a
potentially unsafe situation. Medical devices should
also not interfere with other devices.
In order to regulate the requirements for EMC
(Electromagnetic Compatibility) with the aimto
prevent unsafe product situations, the IEC60601-
1-2 standard has been implemented. This standard
defines the levels of immunity toelectromagnetic
interferences as well as maximum levels of
electromagnetic emissions for medical devices.
Medical devices manufactured for Innovo Groups
conform tothis IEC60601-1-2:2007 standard for
bothimmunity and emissions.
Nevertheless, special precautions need to
be observed:
nThe use of accessories other than those
specified by Innovo Groups,may result in
increased emission or decreased immunity of
the device.
nRefer to the EMC table guidance regarding the
EMC environment in which the device
should be used.
28 29
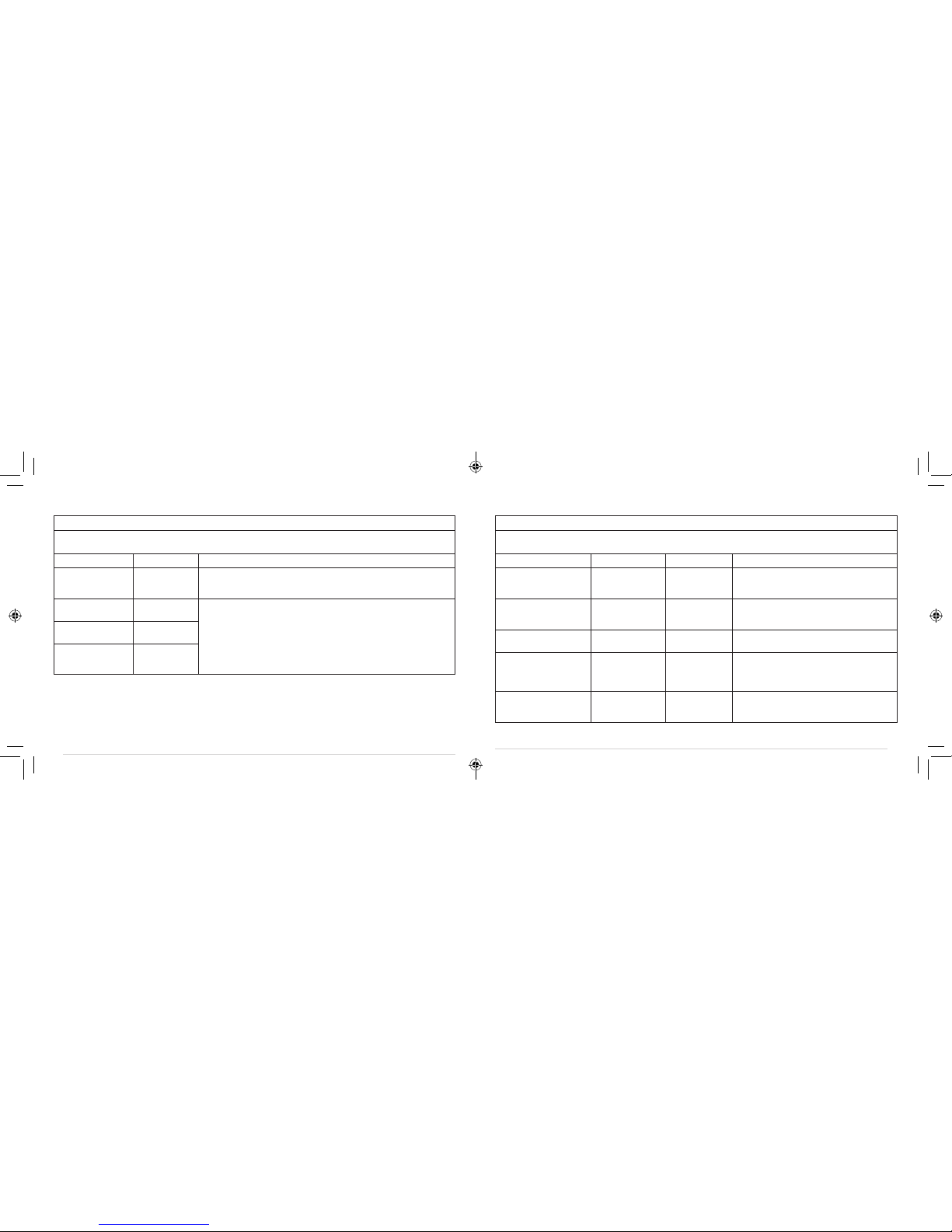
GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC EMISSIONS
iSoothe
™
electrical stimulators are intended for use in the electromagnetic environment specified below. The
customer or the user of these electrical stimulators should assure that it is used insuch environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1 The device uses RF energy only for its internal function. Therefore, its RF
emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR11
Class B The device is suitable for use in all establishments other than domestic and those
directly connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions
lEC 61000-3-2
Not applicable
Voltage fluctuations/
flicker emissions
lEC 61000-3-3
Not applicable
TABLE 1:
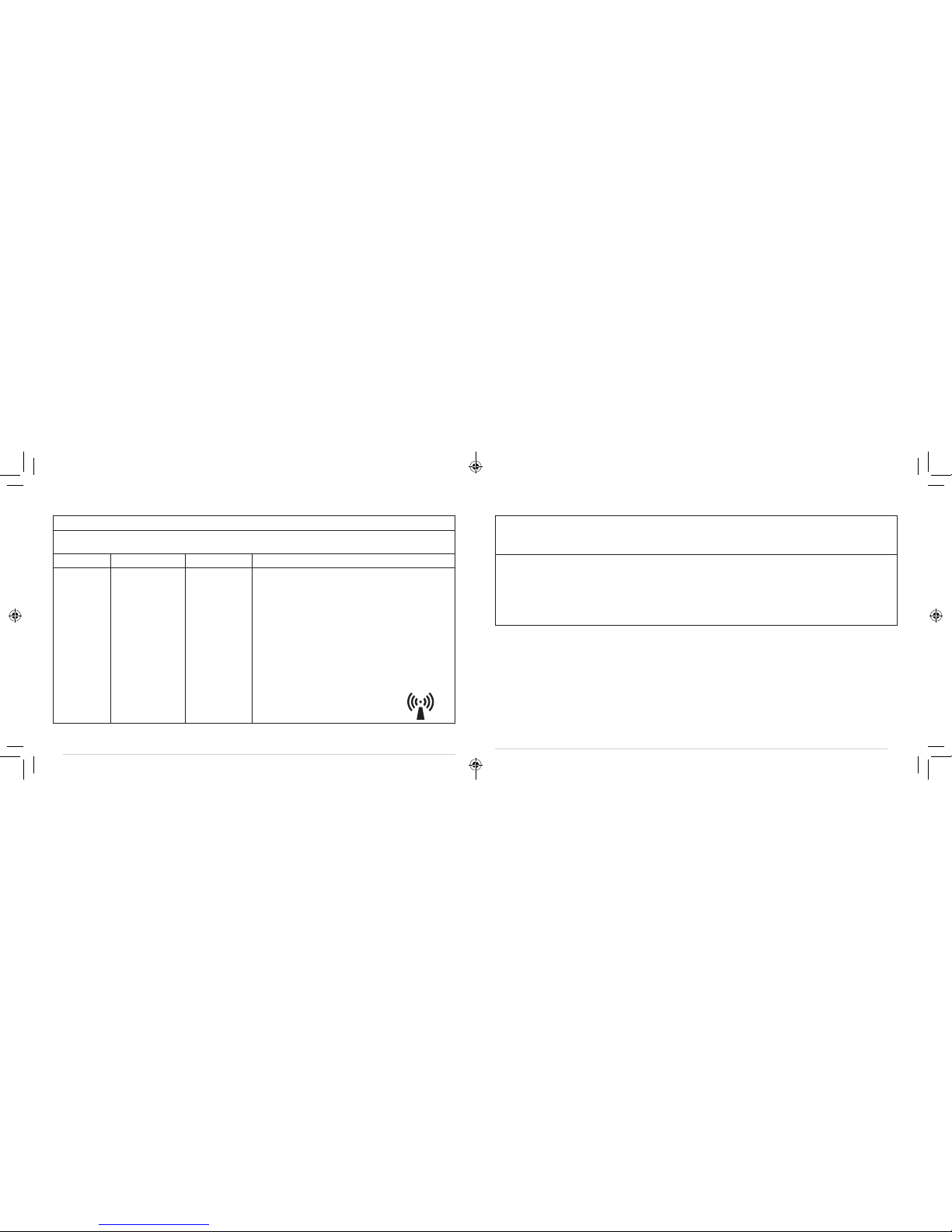
GUIDANCE AND MANUFACTURER’S DECLARATION — ELECTROMAGNETIC IMMUNITY
iSoothe
™
electrical stimulators are intended for use in the electromagnetic environment specified below.
The customer or the user of these electrical stimulators should assure that it is used in such environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
lEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should be wood, concrete or ceramic tile.
If floors are covered with synthetic material, the
relative humidity should be at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
Not applicable Not applicable Not applicable
Surge
IEC 61000-4-5
Not applicable Not applicable Not applicable
Voltage dips, short
interruptions and voltage
variations on power supply
input lines IEC 61000-4-11
Not applicable Not applicable Not applicable
Power frequency
(50/60Hz) magnetic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at
levels characteristic of a typical location in a typical
commercial or hospital environment.
TABLE 2:
30 31
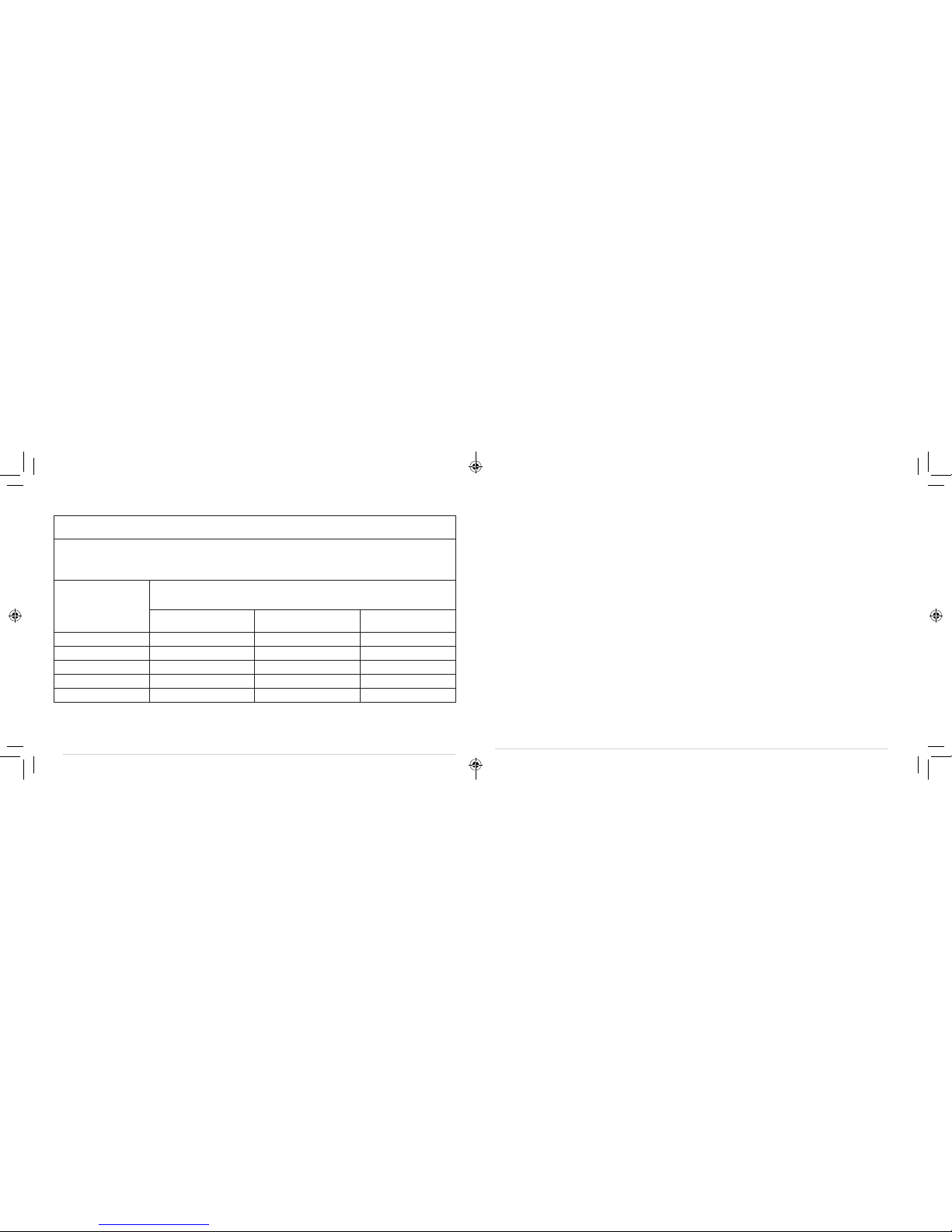
GUIDANCE AND MANUFACTURER’S DECLARATION – ELECTROMAGNETIC IMMUNITY
iSoothe
™
electrical stimulators are intended for use in the electromagnetic environment specified below.
The customer or the user of these electrical stimulators should assure that it is used in such environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF
lEC 61000-4-6
Radiated RF
lEC 61000-4-3
Not applicable
3 V/m
80 MHz to 2.5 GHz
3 V/m
Portable and mobile RF
Communications equipment should be used no closer to any
part of the device, including cables, than the recommended
separation distance calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance
d = 1.2 √P
d = 1.2 √P80 MHz to 800 MHz
d = 2.3 √P800 MHz to 2.5 GHz
Where P is the maximum output power rating of the transmitter
in watts (W) according to the. Transmitter manufacturer and d is
the recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined
by an electromagnetic site survey,ashould be less than the
compliance level in each frequency range. b
Interference may occur in the vicinity of
equipment marked with the following symbol:
TABLE 3:
NOTE I At 80 MHz ends 800 MHz. the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
aField strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess
the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the
measured field strength in the location in which the device is used exceeds the applicable RF compliance level above, should
be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as
reorienting or relocating the device.
bOver the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
32 33
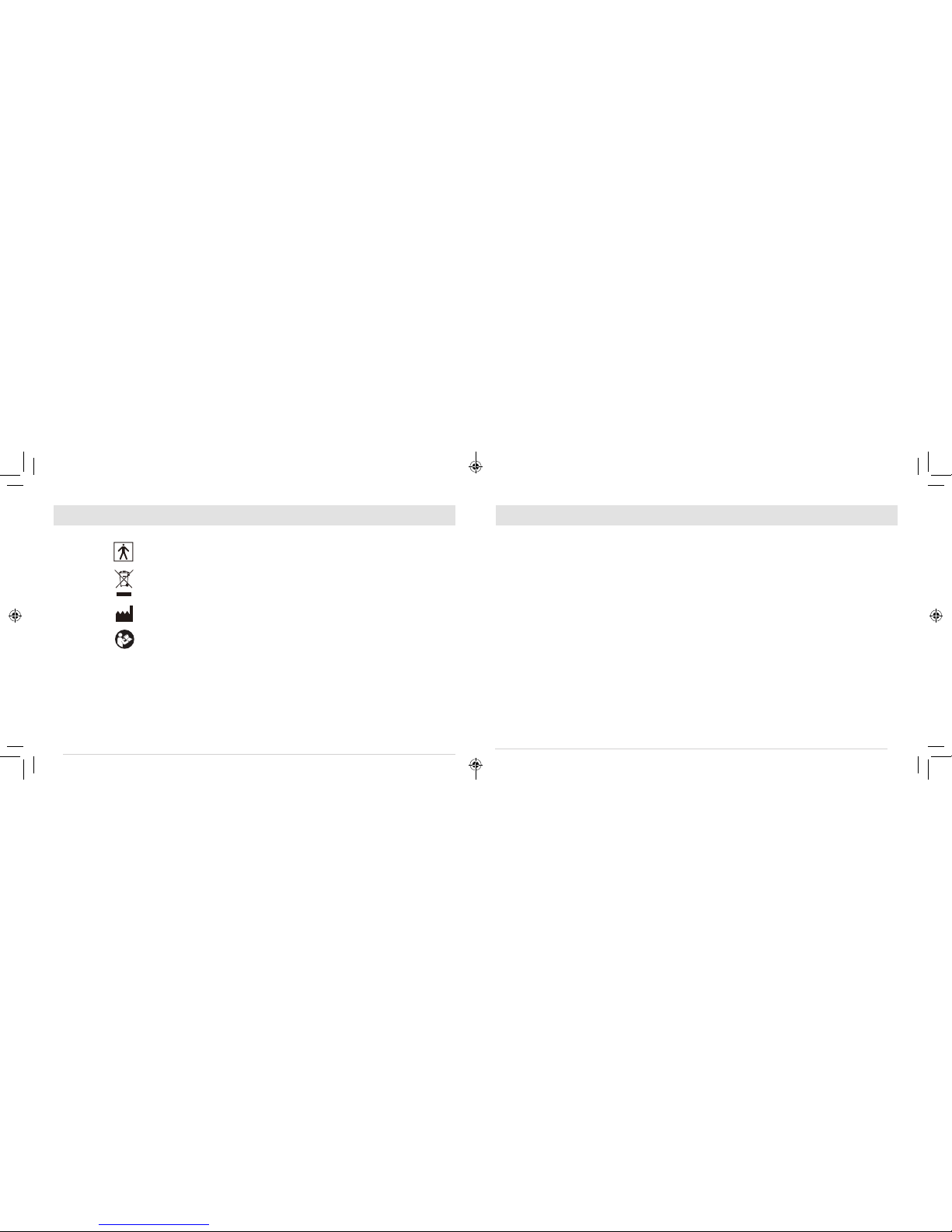
RECOMMENDED SEPARATION DISTANCES BETWEEN
PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT AND THE DEVICE
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled.
The customer or the user of the device can help prevent electromagnetic interference by maintaining a minimum
distance between portable and mobile RF communications equipment (transmitters) and as recommended below,
according to the maximum output power of the communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d = 1.2 √
80 MHz to 800 MHz
d = 1.2 √P
800 MHz to 2.5 GHz
d = 2.3 √P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
11.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
TABLE 4:
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE I At 80 MHz and 800 MHz. the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from
structures, objects and people.
Note: EMC tests conducted including attached electrode cord of 1.5 m length.
34 35
EXPLANATION OF SYMBOLS
Applied part of type BF
Disposal in accordance with Directive 2002/96
EC (WEEE)
The name and the address of the manufacturer
Refer to Instruction Manual.
WARRANTY
You will be required to register your product for
warranty. Visit our website www.innovogroups.com
for more information. You will have to enclose a
copy of your receipt.The following warranty
terms apply:
1. The warranty period for the device is one year
from date of purchase. In case of a warranty
claim, the date of purchase has to be proven by
means of the sales receipt or invoice.
2. Repairs under warranty do not extend the
warranty period either for the device or for the
replacement parts.
3. The following is excluded under the warranty:
nAll damage due to improper treatment,
e.g. non-observance of the user instruction.
nAll damage due to repairs or tampering by the
customer or unauthorized third parties.
nDamage during transport from the
manufacturer to the consumer or during
transport to the service center.
nThe battery and gel pads which are subjected to
normal wear and tear.
4. Liability for direct or indirect consequential
losses caused by the unit is excluded even if the
damage to the unit is accepted as a warranty
claim.
36 37
LIMITED ONE YEAR WARRANTY LIMITED ONE YEAR WARRANTY (CONT.)
Limited Consumer Product Warranty (United States)
This iSoothe
™
Wireless Rechargeable TENS is warranted to the original consumer "the purchaser" to be free from defects in material and workmanship
which are not commercially acceptable for the period of one year from the date of purchase. Warranty coverage terminates if yousell orotherwise transfer
this product to another person. This warranty gives you specificlegal rights and you may also have other rights,whichvary by location.
INNOVO GROUPS MAKES NO EXPRESS WARRANTY OFANY KIND REGARDING THISPRODUCT OTHER THAN THOSE WARRANTIES SET FORTH
HEREIN. ANY IMPLIED WARRANTY, INCLUDING ANY IMPLIED WARRANTY OF MERCHANTABILITY OR ANY IMPLIED WARRANTY OFFITNESSFOR A
PARTICULAR PURPOSE TO THE EXTENT PERMITTED BY LAW, SHALL BE LIMITED IN DURATION TO APERIOD OF 120 DAYS FROM THE DATE OF
PURCHASE BY THE ORIGINAL PURCHASER.
In the event that this product is found by INNOVO GROUPS to not meet the above limited warranty, as purchaser's sole and exclusive remedy, INNOVO
GROUPS
will repair or at the discretion of INNOVO GROUPS, replace this product without charge for such replacement parts or labor. The purchaser shall
bear all expenses related to returning this product to INNOVO GROUPS.
This warranty does not apply to any part of the product that has been subject to misuse, abuse, or alteration. Improperor incorrectly performed
maintenance or repair voids this warranty.
TO THE EXTENT PERMITTED BY LAW, INNOVO GROUPS SHALL NOT BE LIABLE FOR ANY INCIDENTAL OR CONSEQUENTIAL DAMAGESINCLUDING,
BUT NOT LIMITED TO, REPLACEMENT COSTS RESULTING FROM THE BREACH OF ANY WRITTEN OR IMPLIED WARRANTY.
If you wish to make aclaim under this warranty, please contact [email protected] or Tel: +1-858-888-9781.
You will need the following information when making your claim:
nThe entire original iSoothe
™
product and packaging
nThe original receipt showing date of purchase
nDetailed description of the problem
iSoothe™ Model: _____________________________________________
Serial Number: _____________________________________________________
Date of Purchase: ___________________________________________
Distributor: ________________________________________________________
38
Table of contents

















