Inspiration Tecotherm Neo User manual

IfU TECOTHERM NEO TN300 EN - 01.doc
TEC COM GmbH
TECOTHERM NEO
MEDICAL EQUIPMENT for HYPOTHERMIA
of NEONATE and INFANTS
Instructions for Use

IfU TECOTHERM NEO TN300 EN - 01.doc Page 2 of 76 Ref No. TN300 EN-01
1. Contents and abbreviations
page
2. Preface ............................................................................................ 4
2.1 Intended Use ................................................................................... 4
2.2 About Contraindications................................................................... 5
2.3 Operator Profile ............................................................................... 5
3. Informations for Customers, Service & Technical support............... 6
4. Short description of the system........................................................ 7
5. Symbols, Indications........................................................................ 9
6. Warnings , precaution guidelines.................................................... 10
7. TECOTHERM NEO Hypothermia System ..................................... 15
7.1 General description of the system, Operation modes...................... 15
7.2 TECOTHERM NEO device Informations, Performance.................. 26
7.3 Indicators and Operation elements.................................................. 30
7.4 Temperature Probes........................................................................ 33
7.5 Hoses / Hydraulic lines.................................................................... 34
7.6 Fill- Up set ...................................................................................... 35
7.7. TECOmed circulation fluid............................................................... 35
7.8 Mattresses, Cool Wraps, Cool Pads, parts and protective layers ... 36
7.9 MENU, operator interfaces, USB port.............................................. 38
8. NEO Hypothermia System Putting into operationTECOTHERM..... 40
8.1 Initial Set up / Initial Operation ........................................................ 40
8.2 Initial Operation by the customer: Pre- operation Check up........... 40
8.3 Initial Operation by the customer..................................................... 40
8.4 Stop operation/ Turn off device........................................................ 48
8.5 Filling / Refilling Procedures............................................................ 49
8.6 Preparation of empty or partially filled mattress............................... 49
8.7 Application of mattresses................................................................. 50
9. Hygienic Requirements.................................................................... 53
9.1 Cleaning and Disinfecting TECOTHERM NEO................................ 53
9.2 Mattresses, thermally insulated hoses, tubing................................. 53
9.3 Temperature Probes........................................................................ 54
10. Storage............................................................................................ 54
10.1 Storage of the TECOTHERM NEO device....................................... 54
10.2 Storage of Mattresses...................................................................... 55
10.3 Storage of TECOmed ...................................................................... 55
10.4 Transport ......................................................................................... 55
11. Alarms - Malfunctions - Failure - Signals and Troubleshooting....... 56
11.1 System Alarm, System failure ......................................................... 58
11.2 Temperature Alarm.......................................................................... 61
11.3 Alarm No Flow ................................................................................. 64
11.4 Alarm Fluid level too low............................................................... 66
11.5 Alarm No Mains Power ................................................................... 68
11.6 Fluid escapes from the TECOTHERM NEO System....................... 70
11.7 Fault Software Update Error.......................................................... 71

IfU TECOTHERM NEO TN300 EN - 01.doc Page 3 of 76 Ref No. TN300 EN-01
12. Training & Qualification of personnel .............................................. 72
13. Service, preventive maintenance, Software Update........................ 72
13.1 Service & Maintenance.................................................................... 72
13.2 Cleaning the ventilation hole structure (device bottom)................... 72
13.3 Substitution of TECOmed fluid ........................................................ 72
13.4 Substitution of TECOmed fluid in mattresses and cool wraps......... 73
13.5 Software Update.............................................................................. 73
13.6 Check/ calibration of temperature probes........................................ 73
13.7 Safety, Reliability............................................................................. 74
14. Technical Data, TECOTHERM NEO Specification.......................... 75
15. Declaration of Conformity................................................................ 76
16. Disposal........................................................................................... 76
Abbreviations
IfU Instruction for Use
BCT Body Core Temperature
SF System Failure

IfU TECOTHERM NEO TN300 EN - 01.doc Page 4 of 76 Ref No. TN300 EN-01
2. Preface
2.1 Intended Use
This Instructions for Use (IfU) presents a detailed introduction into the
operation modes of TECOTHERM NEO from TEC COM GmbH.
The Hypothermia System TECOTHERM NEO is designed for controlled
comfortable cold & heat treatment procedures. By means of mattresses or
cool wraps cold and heat is applied to the total body, body parts or areas of
neonate depending on the therapy objective.
One main application is induced hypothermia treatment of neonate affected
with Hypoxic Ischemic Encephalopathy.
The IfU contains a Technical Desription and technical data.
TECOTHERM NEO of TEC COM exhibits SERVO CONTROL operation as
a modern excellence feature using MENU assistance.
TECOTHERM NEO of TEC COM has been equipped with two micro
computers and with numerous monitoring and alarm features which
guarantee a high standard of treatment and operation safety.
We highly recommend that the operator is familiar with the operation modes
and capabilities of the TECOTHERM NEO. Prior to putting into operation
carefully read these IfU!
This IfU is associated with a software version. The valid version number is
displayed at the lower left part of operator’s MENU display screen after
switching on the device.
Note: The Manufacturer carries responsibility for basic safety, reliability and
capability of the TECOTHERM NEO system only when
local electrical installation fully meets the requirements of the IfU.
initial operation is performed according prescribed Instruction
procedure by authorized personnel.
TECOTHERM NEO is operated according to the instructions and
statements of said IfU.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 5 of 76 Ref No. TN300 EN-01
2.2 Contraindications for Use
No general contraindications are known. For possible adverse effects study
the relevant treatment and therapy protocols.
Avoid direct contact of mattress or cool wrap with patient’s skin!
Avoid direct contact of mattress or cool wrap with fresh or non- closed
wounds, infectious areas, areas with ulceration and abcesses, rash and
burns.
2.3 Requirements: Operators Profile
Operating a TECOTHERM NEO requires
Healthcare Professional experienced in using life support and life
sustaining equipment
Personnel must be trained in the use of the TECOTHERM NEO
before operating the device.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 6 of 76 Ref No. TN300 EN-01
3. Informations for Customers
Service & Technical support
For Technical Support in German please contact:
TEC COM GmbH
Phone +49 - 345 - 120 52 04
Fax +49 - 345 – 120 52 11
E mail info@teccom-halle.de
For Technical Support in English please contact:
Inspiration Healthcare Ltd
Phone +44 - 1455 840555
Fax +44 - 1455 841464
E mail info@inspiration-healthcare.co.uk
The manufacturer TEC COM or authorized representives will instruct and
introduce the operation personnel prior putting the equipment into operation
Information, technical support, additional manuals may be requested from
any authorized distribution partner.
Manufacturer TEC COM GmbH
Gesellschaft für Technik, Technologie und Vermarktung
Böllberger Weg 170
D-06128 Halle/Saale
Germany
Type label
TECOTHERM NEO
Seriennummer 2010/07/01
230 V 50 Hz 1,5 A
Si 2 x T 2,5 A / 250 V Schutzklasse I IP 20
TEC COM GmbH
Gesellschaft für Technik, Technologie und Vermarktung
Böllberger Weg 170 D-06128 Halle/Saale
Made in Germany
0494 DIN EN 60601-1, DIN EN 60601-1-2

IfU TECOTHERM NEO TN300 EN - 01.doc Page 7 of 76 Ref No. TN300 EN-01
4. Short Description of the Equipment
The Hypothermia System TECOTHERM NEO is designed for controlled cold & heat
treatment procedures and application of specific cold and heat doses to neonate and
babies. The system applies cold and heat to total body, body parts and areas
depending on therapy target by means of mattresses and / or cool wraps NEO PAD.
One main application is hypothermia treatment of neonate affected with Hypoxic
Ischemic Encephalopathy.
Another application designed for controlled hypothermia treatment is Total Body
Treatment of Children up to body mass of 50 kg.
TECOTHERM NEO consists of a unique cold & heat generating device, applied parts
like mattresses and wrap, interconnecting hoses (tubing set), accessories. Applied
parts are connected to the device via hoses by self- sealing quick- disconnect
couplings.
The patient is provided with cold and heat according therapy target in a fully controlled
way by a circulating fluid. This physiologically safe water- based fluid is cooled or
warmed in the device and flows through the mattress or wrap continuously supplying
the patient with therapeutically prescribed doses.
Patient temperatures are measured with approved calibrated probes connected to the
TECOTHERM NEO device. Temperature data is permanently communicated to the
device Operational System.
Circulation of thermalizing fluid to provide cold and heat, accurately reaching set points
and operating at set point temperatures accurately with max. deviation of +/-0,3°C,
monitoring the treatment, and alarming when when exceeding or falling below
temperature limits are Essential Performances of TECOTHERM NEO.
TECOTHERM NEO is electronically divided into a Operational System and a
Controlling System. Both sub systems are micro computer (µC) based. Both µC
communicate permanently to ensure safe and proper operation according to therapy
needs.
A comfortable user MENU will guide the operator to the treatment modes, advise how
to proceed the treatment and how to manage treatment details.
Menu language may be preselected using Main Menu: English, Deutsch, Espanol …. .

IfU TECOTHERM NEO TN300 EN - 01.doc Page 8 of 76 Ref No. TN300 EN-01
TECOTHERM NEO offers three separate treatment modes to induce hypothermia and
to re- warm the patients. TECOTHERM NEO uses 2 independent temperature probes:
rectal probe for measuring Body Core Temperature (BCT) and
skin probe for measuring skin abdominal or forehead temperature etc
The three treatment modes are
(1) SERVO CONTROL Programmable Complete Treatment Mode
(2) SERVO CONTROL Constant Rectal Temperature Mode
(3) Constant Mattress Temperature Mode
The Operator selects the treatment mode 1, 2 or 3 and treatment parameters for
inducing hypothermia, normothermia or hyperthermia following the MENU instructions.
Attention: Temperature probes must be properly placed before treatment procedure
can start.
Treatment and selection of treatment procedure is fully within the responsibility of the
therapist, physician or trained qualified medical personnel.
Attention For neonate and other patient hypersensitivity or restricted compatibility
to hypothermia and / or hyperthermia is in general not known. Neonate receiving such
treatment must be under careful observation.
IfU TECOTHERM NEO TN300 EN - 01.doc Page 9 of 76 Ref No. TN300 EN-01
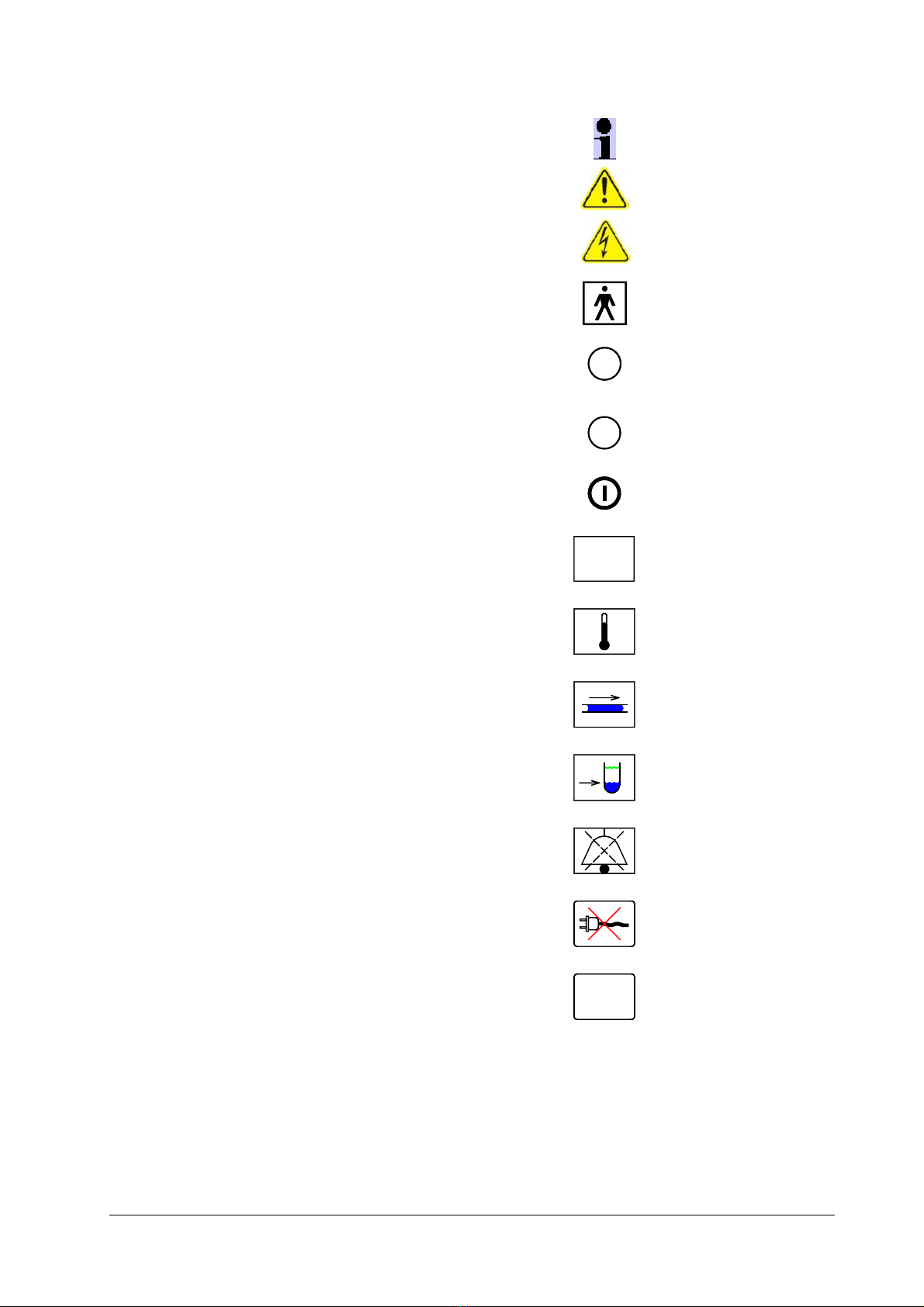
5. Symbols, Indications
Important Information
Attention, Caution, Warning
Electrical Hazard !
Applied Part Type BF
Rectal Temperature Sensor socket
R
Skin Temperature Sensor socket
S
Key “Turn On”
System failure.
SF
Temperature Alarm
Alarm No or restricted Flow.
Alarm Low fluid level.
Symbol AUDIO paused
No Mains Power
Internal System Failure
SF

IfU TECOTHERM NEO TN300 EN - 01.doc Page 10 of 76 Ref No. TN300 EN-01
6. Warnings, Precaution guidelines
Warnings
Do not open the device!
Risk of electrical shock. Opening the device is restricted to service and other
authorized personnel.
Do not remove cover part. Risk of electrical shock when touching inner parts and
components
The TECOTHERM NEO device must be plugged to the mains only to shockproof
sockets. Mains voltage must be 230 VAC/ 50 Hz or 110 VAC/ 60 Hz. Use only
cord supplied with the device or a medical grade approved equivalent cord not
longer than 2,5 m.
Repair and maintenance are restricted to authorised personnel only!
For a reliable and safe operation use only original components, applied parts
and spare parts supplied or recommended by the manufacturer.
Only TECOmed (or approved substitute ) should be used as circulating fluid.
Use only temperature probes in accordance with IfU and with the technical
specification of the manufacturer. Applying different probes may lead to incorrect
and wrong temperature data.
This is likely to put patients at significant risk!
Ensure that Rectal and Skin Temperature Probes are correctly placed in / on
the patient and that probes are properly secured. Also ensure that probes
properly connected to the TECOTHERM NEO socket marked "R" (Rectal for
Body Core Temperature) and “S” (Skin for Surface Temperature).
The same holds for bladder temperature probes measuring children’s BCT.
Use probes with the correct mating connector to the “R” socket.
Caution
When TECOTHERM NEO is run in the Constant Mattress Temperature Mode to lower
body core temperature an independent temperature measurement is required to
monitor hypothermia.
Note that applied treatment temperature and duration do not allow a realistic estimation
of the actual degree of lowering of patient’s BCT.
Medical electrical equipment needs special precautions regarding Electromagnetic
Compatibility and needs to be installed and put into service according to the
Electromagnetic Compatibility information provided in the Technical Description and
Service & Maintenance Manual.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 11 of 76 Ref No. TN300 EN-01
Portable and mobile RF communication equipment can affect medical electrical
equipment. Observe the recommended separation distances listed in the Technical
Description.
The TECOTHERM NEO device should be subject to regular maintenance and
service, see section 13
Fill and replace fluid regularly every 3 months, see section 8.5.
Only TECOmed (or approved substitute) should be used.
Note Circulation may stop, fluid flow stops.
In such cases mattresses may cool neonate or patient slowly down. Especially
during treatment re-warming phase neonate may suffer from extraction of body heat
back into the mattress. Change such condition soon!
The user should not apply other cleaning, disinfecting and decontamination
procedures than those recommended by the manufacturer. If in doubt contact your
local representative.
Precaution Notes for placement
Location
The unit must be placed horizontally onto a level support
The system is fan cooled. Sufficient space must be allocated so that a free flow of air
from all sides can reach the bottom of the device when it is in operation.
Do not place the device into small cabinet compartment or onto small scale boards.
Do not cover the device!
Device should be located so that there is a distance of at least 15cm between rear
side and a wall or another limiting surface to ensure free outflow of the cooling air.
The unit should be placed avoiding air to be blown towards the patient.
The unit should be placed so that optical blink alarms are clearly seen and acoustic
alarms are clearly audible.
Do not place mattresses and hoses onto hot or warm supports during operation
Do not place the device during operation near intensive heat sources.
Attention Ensure and leave enough space around the TECOTHERM NEO not to
obstruct passage of acting personnel. Ensure that hoses, cable cord, temperature
probes etc. do not form obstacles.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 12 of 76 Ref No. TN300 EN-01
Ensure that placement of TECOTHERM NEO is not forming trapping zones for
hands and fingers to avoid contusions and other injuries.
Check type of mattress and interlayer. Use thin disposable interlayer which must at
least fully cover the mattress with some border. Such interlayer must be coated at
lower side with plastic coating to prevent penetration of blood, liquids or liquid media
onto the upper surface of the mattress and to protect the patient.
Note: Place mattress/ cool wrap onto a 10 - 20 mm thick foam material that has
good thermal insulation during operation.
Using an incubator
When using an incubator to perform hypothermia treatment
Pay attention that there is enough space to properly place mattress or cool wrap.
Otherwise kinking of hose set and / or tubing near mattress and mattress folding
may cause restricted fluid flow, bad circulation or even flow blocking
Place the hoses set in a way that the hoses are in a straight line. Fasten the hoses
in such a way to avoid kinking of the tubing near the mattress
Note: Place mattress onto a 10 - 20 mm thick foam material that has good
thermal insulation during operation.
Note: Do not put mattress directly onto compact silicon inlays used in incubators
or the like.
Attention Ensure that incubator heaters are shut off! Ensure that there is no forced
air circulation. It may cool down the neonate in a re- warming phase of the treatment.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 13 of 76 Ref No. TN300 EN-01
Indications for hazardous substances
TECOTHERM NEO does not contain parts or substances stemming from derivatives of
blood or human/ animally tissues.
TECOTHERM NEO does not contain parts made of Latex or its derivatives.
TECOTHERM NEO Applied Parts do not contain parts made of PVC with DEHP
softener/ plasticizer
Thermalizing Fluid TECOmed is made of 70 % water, 30% pure Ethyl alcohol. Very
small quantity < 0,3 % denaturing agent Methyl- Ethyl- Ketone MEK is added. Details
see Safety Data Sheet.
Caution Do not swallow or ingest fluid.
Skin contact with TECOmed fluid is harmless.
Note After use close TECOmed containers tightly
Ambient Conditions
To ensure a proper operation in normal use pay attention to the following conditions
Protection The device should be protected from dampness and wetness
(e.g. splash water.)
To have full cooling power ambient temperature should not exceed 27°C. Otherwise
the TECOTHERM NEO system may not achieve the lowest possible set temperature
of a big mattress.
Relative Humidity during treatment within a range of 30 % - 80 %
Ensure that during treatment/ operation no installations, systems, devices and the
like are operating or are intended to operate next to TECOTHERM NEO and
producing
strong electromagnetic radiation
ultraviolet radiation
intense infrared radiation
mechanical shocks, vibrations.
Do not intensively illuminate the device.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 14 of 76 Ref No. TN300 EN-01
NOTE Information and Advice for operators
Users and Operators should intensively read the I f U to become familiar with all
instructions and technical details
To acquaint operators and users with instructions and measures to handle alarms and
situations under single fault conditions is important.
Operator is requested to become mentally familiar with the treatment equipment as a
physiological closed- loop control system PCLCS *) , see section 7.
Note for operators and users To be familiar with instructions and measures to
handle alarms and situations under single fault conditions is important.
Note for operators and users When placing the TECOTHERM NEO device
positions and motions of patient, operators and other personnel have to be taken into
consideration. Circumstances and situations near TECOTHERM NEO site before,
during and after treatment have to be considered.
Note for operators Consider possible mechanical means for positioning, supporting or
erecting the patient during treatment. They may block or reduce fluid circulation
We recommend that operator should independently monitor patient temperatures
during treatment.
*) PCLCS and Mental Model
To develop an imagination on cause and effect in TECOTHERM NEO treatment mode 1,
Section III, a realistic single fault condition scenario is assumed: Rectal Temperature Sensor
is slipped out the patient’s rectum.
The probe now detects a much lower temperature measuring ambient air temperature near the
neonate rectum. This temperature shown at the display screen strongly deviates from the BCT
33,5 °C (cause). To reduce the deviation TECOTHERM NEO is increasing the fluid / the
mattress temperature (effect): the Control Board responds with activation of warming to
increase the “ pretended BCT, appearently to low “.
After 5 minute period the device indicates temperature alarm. Display is showing confusing
temperature data: Low rectal temperature, normal skin temperature, high mattress
temperature. But a mentally prepared operator decide to immediately inspect and check the
position of the rectal probe.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 15 of 76 Ref No. TN300 EN-01
7. TECOTHERM NEO Hypothermia System
7.1 Description of the System
TECOTHERM NEO Hypothermia system, system components and accessories
TECOTHERM NEO device
Application parts like mattresses, cooling wraps, cooling pads
Temperature probes with connectors to the device
Hose set, thermally shielded to connect application parts to the device
Fill-up set, includes necessary components for filling/ re- filling TECOmed
5 litre TECOmed fluid, canned in PE or PP container
Thin protective interlayer
Electrical Power cord, up to 2,5 m
Optional
Repair set for small mattress defects like punctures and flaws
Fluid Emptying Aid for mattress
Storage boxes
Cooling wraps for total body cooling of neonate and infants for Multiple Use
Mattresses for Total Body Cooling are Therm Aqua Pad 30 x 45 cm (small size) and
50 x 90 cm (medium size)

IfU TECOTHERM NEO TN300 EN - 01.doc Page 16 of 76 Ref No. TN300 EN-01
Interlayer separate mattresses/ wraps from patient body preventing intimate direct
contact.
For total body cooling treatment the TECOTHERM NEO device pumps TECOmed
along the hydraulic lines through mattresses / wraps or pads to lower or increase body
core temperature to the set value. Users may preselect the most appropriate treatment
procedure and target temperatures.
During circulation the micro computer of the Control Board is permanently comparing/
analysing the measured and set rectal temperatures. The larger the deviation between
the two temepratures the more cooling or heating power is to be supplied by the
TECOTHERM NEO device.
Rectal temperature stands for body core temperature BCT.
Treatment procedure temperatures for inducing hypothermia in neonate are strictly limited
Rectal Temperature standing for BCT 32°C/ 35°C
(lower limit / upper limit) in the treatment mode 1
Mattress temperature 12°C/ 39°C
Device internal temperature alarm limits 10°C lower limit
41°C upper limit

IfU TECOTHERM NEO TN300 EN - 01.doc Page 17 of 76 Ref No. TN300 EN-01
Treatment and operation modes of TECOTHERM NEO
TECOTHERM NEO is designed as a physiological closed- loop control system PCLCS ,
see this section.
Three treatment and operation modes of TECOTHERM NEO
1 Programmable Complete Treatment Mode (Servo controlled mode)
2 Servo Control Mode (constant rectal temperature)
3 Constant Mattress Temperature Mode
Note: The operator can permanently follow the Rectal Temperature on the display
screen, see section 7.3.
Treatment Mode 1
Programmable Complete Treatment Mode (Servo controlled mode)
(automatic mode, treatment protocol)
System is designed for inducing hypothermia in a patient by total body cooling. Details
for inititial operation see section 8.
Note This mode is limited to temperatures and
profiles stated in the TOBY Study protocol
(see reference) for inducing hypothermia in neonate
suffering from Hypoxic Ischemic Encephalopathy
Rectal temperatures can be selected and set within a range 32°C to 38°C.
Temperature is accurately adjusted and finely tuned.
Necessary TECOTHERM NEO system equipment to run treatment is
TECOTHERM NEO device
Mattress for total body cooling, filled, protecting foil interlayer
Rectal Temperature Probe, Skin temperature probe
Themally shielded hoses to connect mattress to the device.
All equipment parts should be checked and prepared to start treatment, see section 8
of this IfU. Patient – neonate – should be placed onto the mattress/ cool wrap.
Rectal probe should be inserted and secured.
To start treatment you have to follow and observe the instructions of the MENU.
Operation Mode 1 has five (5) treatment sections/ phases.
temperature
time

IfU TECOTHERM NEO TN300 EN - 01.doc Page 18 of 76 Ref No. TN300 EN-01
Treatment section I (optional) Thermal preparation of the mattress
Prior to start hypothermia treatment you may prepare the mattress thermally. Following
treatment procedure will be performed by the system independently on this section.
Whether such preparation is appropriate depends on your information state on the
neonate you have to treat. A mattress precooled to 18 – 22°C may shorten the cooling-
down treatment section II.
Treatment section II Rapid Cooling- Down Phase
TECOTHERM NEO is operating to cool down as fast as possible. The system is
operating with maximum cooling power. Body heat from the patient/ neonate is
eytracted by the cooling mattress and transferred to the circulating fluid. The central
device cooling unit extracts the heat and cools the fluid on demand.
When rectally measured BCT is reaching the target temperature 33,5°C the system will
reduce the cooling power automatically. Control board microcomputer determined a
sufficiently small deviation of measured and set temperature to start power reduction.
Control system approaches set temperature without remarkably overshooting/
undershooting.
Treatment section III Cooling Phase
Reaching the set temperature 33,5°C the system continues treatment to hold BCT of
the patient constant. Treatment time is preselected by the operator (default 72 h). The
Rectal (BCT) temperature is pre-selected (default 33,5 °C). The control board
permanently compares measured rectal temperature with set BCT 33,5°C. The central
cooling unit is permanently adjusting the temperature of the circulating fluid to hold
BCT at 33,5°C.
If during treatment section III BCT of the patient is increasing/ decreasing caused by
disturbances the difference between measured and set rectal temperature increases.
The system initially will respond with more cooling power or increasing warming.
If deviation exceeds 0,5 °C in any direction, for some longer time the internal
monitoring system will detect this discrepancy. TECOTHERM NEO starts alarming.
Treatment section IV Re- Warming Phase
After cooling phase III the system automatically starts the re- warming phase. Re-warm
target Rectal (BCT) Temperature and time to achieve this are pre-selected (Default
BCT 37°C and 7 hours). The TECOTHERM NEO system will linearly increase the BCT
until selected target temperature 37°C is reached.
System then stops re- warming. BCT remains at the selected value.
In case of disturbances the system is responding as described in section III.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 19 of 76 Ref No. TN300 EN-01
Treatment section V (optional) Holding BCT at Normal Temperature
The operator can continue the treatment. TECOTHERM NEO will automatically
continue to hold neonate at the selected BCT 37°C or at a modified temperature.
Operator can finish such treatment at any time.
Having in mind the PCLCS in this treatment mode 1
Along all treatment phases the Control Board is permanently - every 2 seconds -
analysing temperatures and temperature differences. It instructs the central cooling unit
to cool or warm the circulating fluid depending on the results of this analysis. The
circulating fluid then extracts or transfers thermal energy from/ to the patient.
Circulation is monitored by the Control board.
All temperatures / time dates are recorded/ logged and can be read out/ transferred to
an USB stick, see section 7.3 for the USB port.
NOTE: All parameters can be changed from set position at ANY TIME should the need
arise. Changes will be stored on the TECOTHERM NEO and can be seen on later
analysis
NOTE: All parameters can be changed from set position at ANY TIME should the need
arise. Changes will be stored on the TECOTHERM NEO and can be seen on later
analysis
Attention Fallback Mode
If treatment is interrupted or any reason for stopping operation in Automatic Treatment
Mode 1 you may continue treatment using Treatment Mode 3 Constant Mattress
Temperature which is a manual mode.
Note Such treatment is a true alternative solution to continue treatment under mode 3
conditions (Fallback Case)
Note Study details of treatment mode 3 prior to change treatment, see below.
Note Transition from automatic mode to Treatment Mode 3 Constant Mattress
Temperature must be performed by the operator:
TECOTHERM NEO rectal temperature probe should be disconnected and removed.

IfU TECOTHERM NEO TN300 EN - 01.doc Page 20 of 76 Ref No. TN300 EN-01
BCT monitoring requires installation of another independent accurate calibrated
temperature probe (rectal probe or bladder catheter sensor) with a separate display
showing the rectal temperature.
Note Operator may continue to use the skin sensor connected to port “S” of the
TECOTHERM NEO device as an auxiliary means if no other probes are available.
That temperature is displayed on the screen in the small parameter boxes in the
upper part of the display feature DIAGRAM and as temperature profile in the display
feature DIAGRAM, see section 7.3 of this IfU.
Change to the Main MENU and select treatment mode 3 Constant Mattress
Temperature Mode.
If transition to fallback mode must be done during treatment section II (cooling
down): To cool down fast and efficiently to reach BCT 33,5°C select low mattress
temperatures ( 12 – 15°C). Then follow the instructions of treatment mode 3, see
below this section.
During treatment phase III or IV: Follow instructions of treatment mode 3, see this
section below.
Other manuals for Tecotherm Neo
2
Table of contents
Other Inspiration Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Whitehall
Whitehall Thermalator LITTLE Therm Instructions for operation and care
Nellcor
Nellcor OxiMax N 5500 Service manual
Max-Ability
Max-Ability Pressalit 2100 Installation drawing
ORTHOSERVICE RO+TEN
ORTHOSERVICE RO+TEN malleonite manual

Uniq Medical
Uniq Medical Q 500 Plus Instructions for use
TensCare
TensCare OVA+ Instructions for use