Inspiration Tecotherm Neo User manual
Distributed by:
2/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
Note:
Before use, read this information carefully to familiarize yourself with the system. This is the only
way to ensure safe and proper use of the TECOtherm NEO hypothermia system.
Distributed by:
Inspiration Healthcare Ltd
2 Satellite Business Village, Fleming Way
Crawley, West Sussex RH10 9NE, UK
T+44 (0)1455 840 555
F+44 (0)1455 841 464
Winspiration-healthcare.com

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
3/52
Table of Contents
General information .............................................................................................................6
Signs and symbols .....................................................................................................6
Instructions for safe use............................................................................................7
1.2.1 Indications of hazardous substances....................................................................7
Intended Use Of The Device .............................................................................................8
Intended Use .............................................................................................................8
Contraindications for use/side effects ......................................................................8
Requirements for operators and users......................................................................9
Environmental Requirements....................................................................................9
Introduction and Brief Description .....................................................................................10
System Description .............................................................................................................10
Overview of system components............................................................................10
TECOtherm NEO device...........................................................................................12
4.2.1 TECOtherm NEO –Front Side .............................................................................12
4.2.2 TECOtherm NEO –Rear Side...............................................................................13
4.2.3 Display Menu......................................................................................................14
Power cable.............................................................................................................14
Mattress ..................................................................................................................14
Cover cloth (interlayer) ...........................................................................................16
Hose kit....................................................................................................................16
Refill sets .................................................................................................................17
External temperature sensors.................................................................................17
Overview of the operating modes ......................................................................................18
Description of the operating modes........................................................................19
5.1.1 Automatic operation by program.......................................................................19
5.1.2 Control to constant rectal temperature .............................................................21
5.1.3 Setting to constant mattress temperature.........................................................22
The Fallback Mode –for Patient Safety...................................................................23
5.2.1 Plausibility limits of the rectal temperature.......................................................24
5.2.2 Working method in the fallback mode...............................................................24
5.2.2.1 Fallback mode in cooling phase.....................................................................25

Distributed by:
4/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
5.2.2.2 Fallback mode in the warming phase ............................................................25
Installation of the device and operation.............................................................................26
Commissioning ........................................................................................................27
Filling/Refilling/Emptying of Mattress and TECOtherm NEO...................................27
6.2.1 Filling process .....................................................................................................27
6.2.2 Refill process.......................................................................................................29
6.2.3 Emptying TECOtherm NEO and mattress............................................................29
Commissioning and Operation of the TECOtherm NEO System..............................30
6.3.1 Starting Treatment .............................................................................................32
6.3.2 Monitoring during treatment .............................................................................33
6.3.3 Ending treatment................................................................................................33
6.3.4 Displaying and exporting treatment data...........................................................34
Switching off .......................................................................................................................34
Cleaning ..............................................................................................................................34
Cleaning the outside of the TECOtherm NEO device...............................................35
Cleaning the outside of the mattress and hose kit..................................................35
Cleaning the outside of temperature sensors .........................................................35
8.3.1 Reusable temperature sensors...........................................................................35
8.3.2 Temperature sensors for single use....................................................................35
Transport.............................................................................................................................36
Storage and shelf life ..........................................................................................................36
Troubleshooting..................................................................................................................36
General information on the alarm and monitoring system.....................................36
System alarm...........................................................................................................37
Temperature alarm..................................................................................................38
Flow rate alarm........................................................................................................40
Fluid level alarm ......................................................................................................40
Power Failure/Mains Outage Alarm ........................................................................41
Maintenance and Service....................................................................................................42
General Notes..........................................................................................................42
12.1.1 Preventive maintenance.....................................................................................42
12.1.2 Software updates................................................................................................43
Disposal...............................................................................................................................43

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
5/52
Technical Specifications......................................................................................................43
Electromagnetic Compatibility (EMC) .....................................................................45
14.1.1 Guidelines and manufacturer’s declaration – Electromagnetic interference
emission 45
14.1.2 Guidelines and manufacturer’s declaration – Electromagnetic interference
emission 46
14.1.3 Guidelines and manufacturer’s declaration – Electromagnetic interference
emission 47
14.1.4 Recommended safe distances between portable and mobile HF
telecommunications equipment and TECOtherm NEO ......................................................48
Service Information ............................................................................................................49
I. APPENDIX –Equipment and Accessories............................................................................50
II. APPENDIX –Internal cleaning.............................................................................................51
Distributed by:
6/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
General information
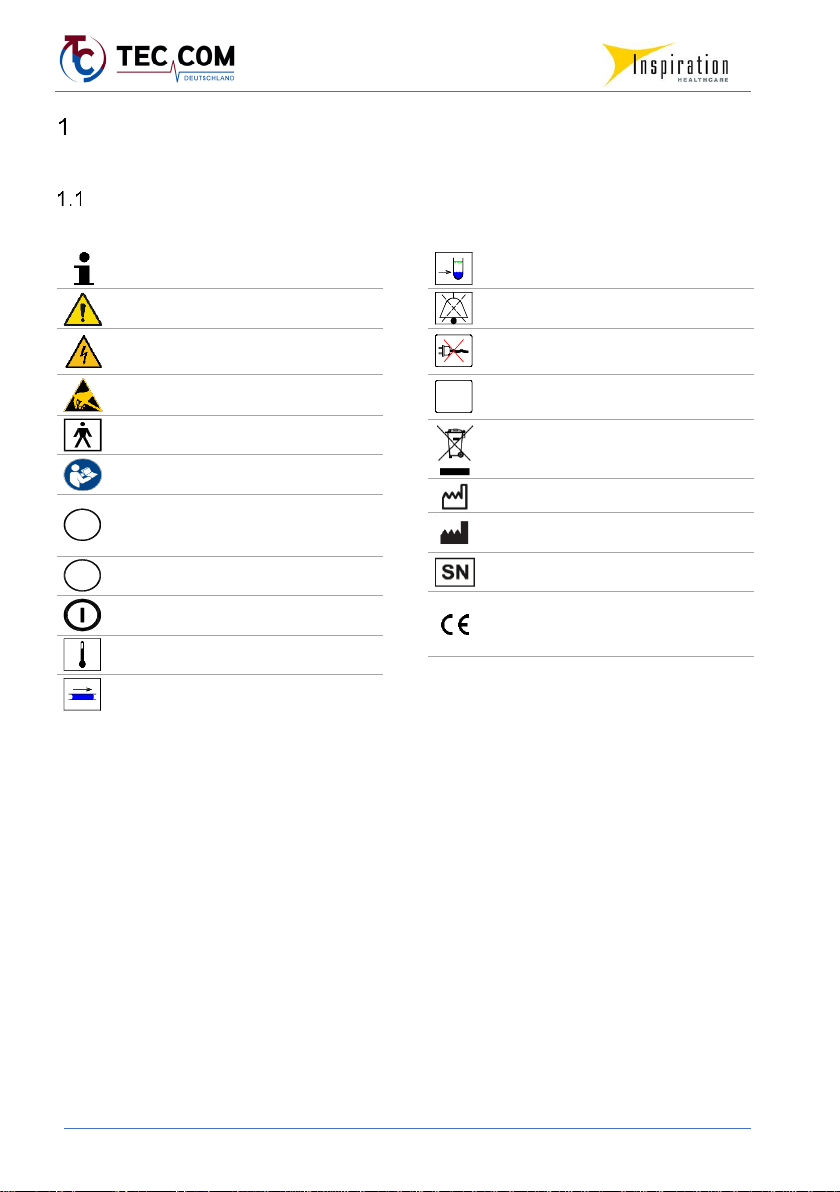
Signs and symbols
Important information
Attention, important notice! Warning!
Caution, electric current!
Caution, do not touch contacts!
Applied part type BF
Follow the instructions for use!
Socket for rectal temperature sensor
Socket for skin temperature sensor
"Switch on" button
Temperature alarm
Flow rate alarm
Fluid level too low
Acoustic alarm paused
Mains failure, power failure
(separate LED display)
Internal system error
(separate LED display)
Do not dispose of with household
waste!
Date of manufacture
Manufacturer
Serial number
CE marking to declare that the product
complies with applicable EU
requirements
R
S
SF

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
7/52
Instructions for safe use
TECOtherm NEO is intended for use by healthcare professionals only.
Modifications to TECOtherm NEO are not allowed!
Only the accessories listed in the Annex and specified for use with
TECOtherm NEO must be used; otherwise correct function is not
guaranteed!
The device may only be opened by authorized personnel. There is a risk of
electric shock.
If the device is in operation, the user must not simultaneously touch the
patient and metallic device parts (e.g., sockets of plug contacts, protective
conductor-connected metal parts of the rear side, or contacts of fuse
holders).
The two sockets for the temperature sensors on the front of the device and
the USB socket on the back are marked with the ESD warning sign. They are
sensitive to discharges of static electricity; their contacts must not be
touched by fingers or tools.
When plugging in the temperature sensors or the USB stick, the following
ESD protection measure is required:
Touch the fan protection grid on the rear of the device with your other hand
first. It is necessary to train all persons working with the TECOtherm NEO
with regard to the importance of the ESD warning sign and ESD protection
measures. In addition to the protective measure prescribed above, this
training should also contain general information on the occurrence, possible
effect and prevention of electrostatic charges.
1.2.1 Indications of hazardous substances
TECOtherm NEO and application parts do not contain any parts of
•derivatives of human blood or human or animal tissue
•Latex
Application parts for TECOtherm NEO do not contain PVC with DEHP plasticizer.

Distributed by:
8/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
Intended Use Of The Device
Intended Use
The TECOtherm NEO hypothermia system is used for the targeted, precisely controlled and
comfortable stationary temperature treatment by means of a mattress, with which the
temperature control (cooling or warming) of the entire body or of body parts takes place as a
function of the therapy objective. The main application is induced hypothermia for the
treatment of hypoxic ischemic encephalopathy (HIE).
The manufacturer assumes responsibility for basic safety, reliability and
performance of the TECOtherm NEO system if:
•The existing electrical installation at the installation site complies with
the requirements of the user manual as well as the legal and normative
requirements.
•The installing and commissioning has been carried out by authorized
personnel.
•The TECOtherm NEO system is operated in accordance with the
instructions for use.
Contraindications for use/side effects
No general contraindications are known. The following instructions must nevertheless be
observed by the user:
Therapeutic whole body hypothermia is a systemic treatment method. Be careful
when choosing the target temperatures during cooling. For re-warming, select
low speeds to slowly bring the body to the core temperature of 37° C.
The heart rate of the patients must be monitored. Patients with cardiac rhythm
disorders or very low heart rate require particularly careful monitoring.
Patients with known hypersensitivity to cooling and warming must only be
treated under supervision.
Open or infected wounds/burns, rashes and other affected regions of the skin
must not come into direct contact with the application parts of TECOtherm NEO.
Cooling can cause shivering to a patient. Shivering counteracts the cooling of the
body. The device registers this, controls against it and reports an alarm if
necessary.

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
9/52
Requirements for operators and users
TECOtherm NEO is intended for use by healthcare professionals only.
The TECOtherm NEO may only be used and operated by persons who meet the following
qualifications and requirements:
•Medical training
•Work experience in intensive care, especially for use in neonatological and child
intensive care units (ICU)
•Experience in dealing with medical-electrical devices and systems
•Instruction and training in the use of TECOtherm NEO before commencing treatment
by the manufacturer or an authorized representative. The sales and service partners
provide instruction and training measures and inform about necessary updates of the
software. Information is also provided about design updates and technical
improvements.
As an operator or user, you must be familiar with the TECOtherm NEO operation
and possible troubleshooting BEFORE USE. Read the instructions carefully. Users
must be familiar with the modes of operation and procedures of hypothermia
treatment.
The user is required to carefully check the data entered or selected in the menu
for correctness and appropriateness before starting hypothermic treatment.
The user is responsible for setting a suitable mode and for setting the required
parameters of the treatment.
Environmental Requirements
For proper operation, the following conditions must be observed during proper use:
The room temperature should not exceed 27 °C or fall below 5 °C for prolonged
time periods. Otherwise, the lowest adjustable temperature is no longer reached,
particularly in the case of large mattresses. The system no longer achieves its full
performance.
The device must be protected against moisture (e.g., splashes) and protected
against humidity and splashes during operation.
The permissible relative humidity during treatment is 30%–80%.
The device must not be operated in rooms where combustible gas mixtures
occur, e.g., anesthetic with oxygen or nitrous oxide N2O.
Distributed by:
10/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
In the vicinity of the TECOtherm NEO, no equipment, devices and equipment may
be in operation or put into operation during treatment that:
•Generate ultraviolet radiation
•Generate infrared radiation
•Generate strong electromagnetic interference with high intensity (e.g., HF
surgery equipment or magnetic resonance tomographs)
•Generate or trigger mechanical shocks or shaking or strong vibrations
Introduction and Brief Description
The TECOtherm NEO device pumps temperature regulation fluid (temperature control medium)
circulating from the device through heat-insulated hoses to the treatment mattress and back
into the device. The aim is to bring the core temperature of the patient wrapped in the mattress
to the target temperature. The ACTUAL body core temperature is measured via a rectal
temperature sensor and compared with the SETPOINT temperature. Depending on the deviation
of the ACTUAL temperature from the SETPOINT temperature and the heating or cooling capacity
of the device, the temperature of the circulating fluid is automatically adjusted by the
TECOtherm Neo.
If necessary, the skin temperature of the patient can be monitored with the aid of an additional
skin temperature sensor. However, the skin temperature is not a control variable for the control
system.
Target temperatures and treatment times can be user defined within certain limits (cf. “14
Technical Specifications“).
System Description
Overview of system components
The TECOtherm NEO hypothermia system consists of at least the following components:
Designation
Designation
TECOtherm NEO device,
incl. installed software
Sets and controls temperature and data storage
Power cable
Connects the device to the mains
Mattress
Serves patient temperature
Constant-temperature
medium
Flows through the mattress, serves for temperature control
Hose kit
Connects the mattress to the device, creating a circulating
fluid cycle.

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
11/52
Designation
Designation
Refill sets
Used to fill the system with temperature regulation fluid
External rectal temperature
sensor
Controls and checks the core body temperature
Optionally, the following accessories can also be used:
Designation
Description
Fleece cover cloth
(for reusable mattresses)
Used to protect reusable mattress or patient (see section
4.5 Cover cloth (interlayer))
Mattress repair set
Set for repairing minor defects on the mattress
External skin temperature
sensor
Used for optional skin temperature measurement
Only the accessories specified in “I APPENDIX –Equipment and Accessories“
must be used with TECOtherm NEO. This is the only way to ensure safe
operation of the hypothermic system!
Distributed by:
12/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
TECOtherm NEO device
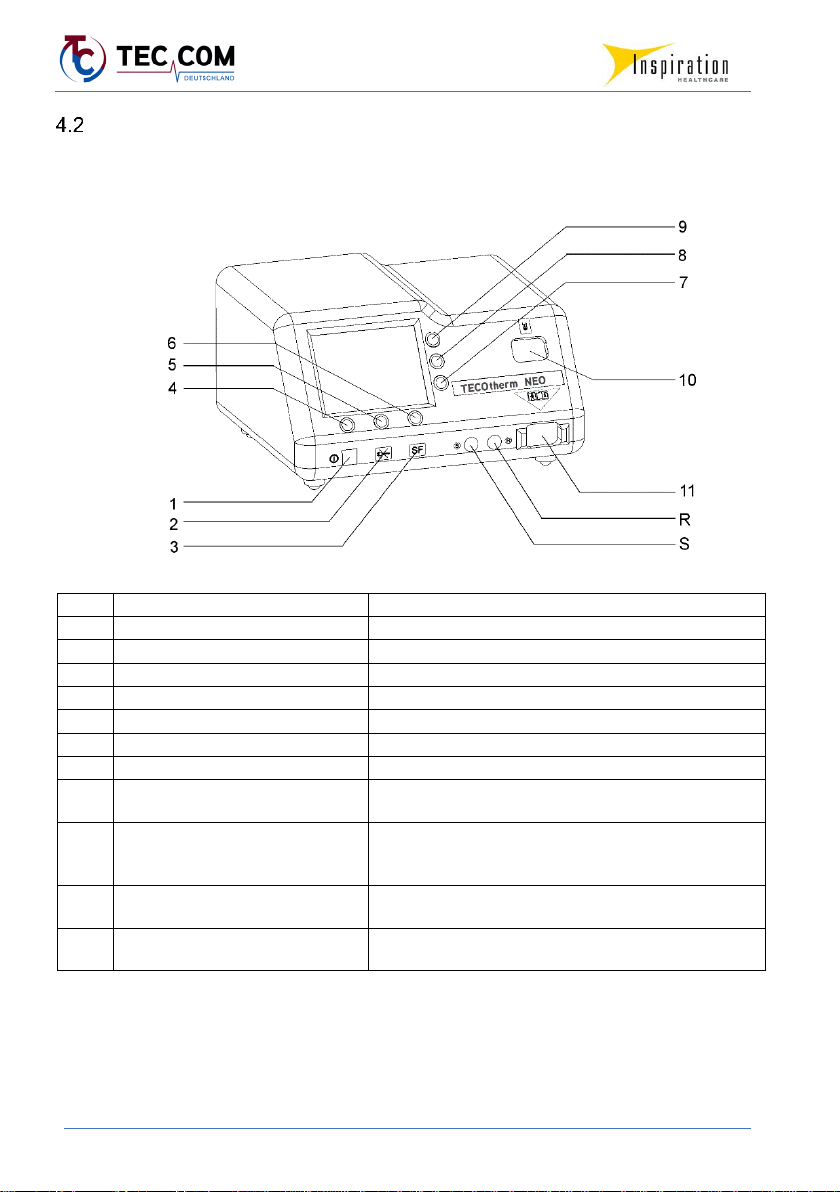
4.2.1 TECOtherm NEO –Front Side
No.
Designation
Description
1
Switch-on button
Switches the device on.
2
Power failure status LED
lights in case of power failure
3
“System error” status LED
lights in case of system errors
4 - 6
Function keys
Execute the action options displayed on the screen
7
Key for menu arrow ▼
Moves cursor down or decreases numerical value
8
“Alarm mute” key
Mutes the acoustic alarm temporarily
9
Key for menu arrow ▲
Moves cursor up or increases numerical value
10
Coupling connectors for refill set
Serves for connecting the refill set for filling/refilling
temperature regulation fluid
11
Coupling connectors for hose kit
Used to connect a hose kit. The liquid flows out of the
device via the left connection and returns to the device
via the right connection.
R
Connector socket for rectal
temperature sensor (R= Rectal)
Used to connect a rectal temperature sensor
S
Socket for skin temperature sensor
(S = skin)
Used to connect a skin temperature sensor
Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
13/52
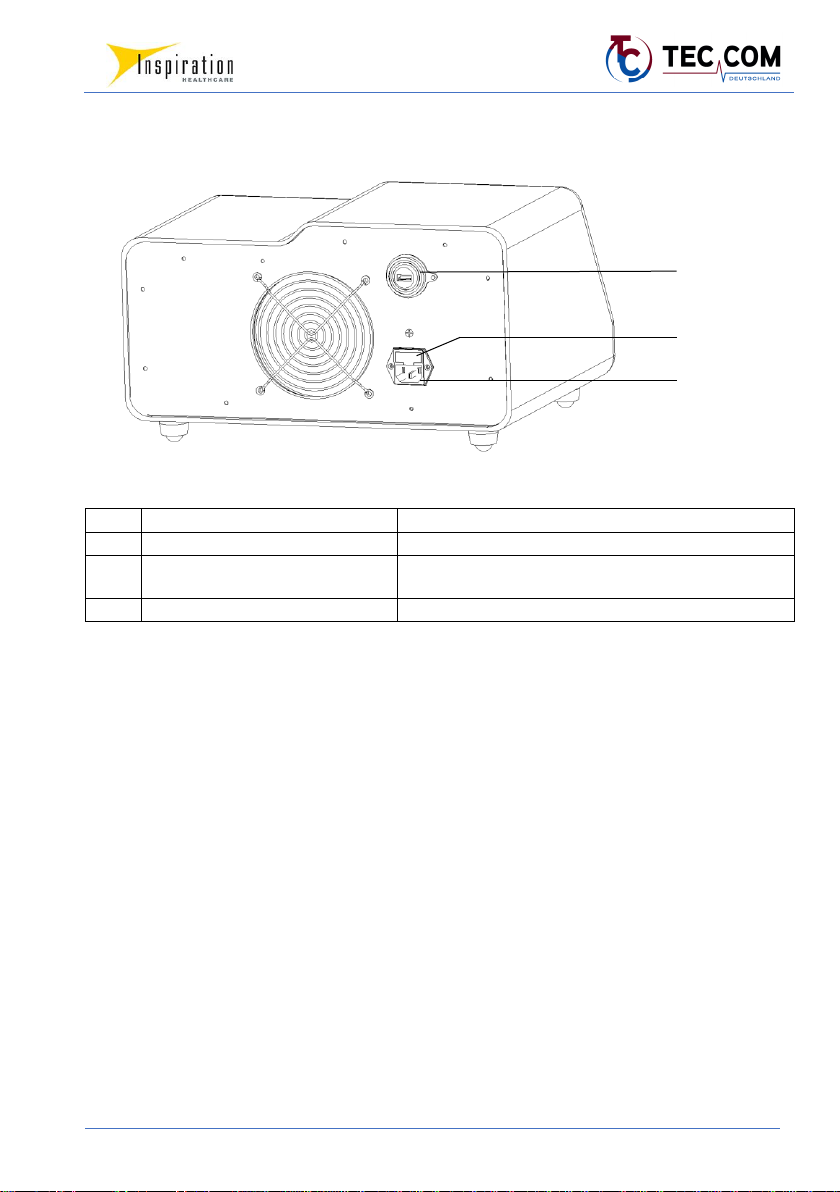
4.2.2 TECOtherm NEO –Rear Side
No.
Designation
Description
12
USB port
Connects a USB stick to store data
13
Fuses
behind the panel of the mains plug socket: 2 replaceable
fuses (cf. type plate)
14
Mains plug socket
Connects a power cord to the power supply
12
13
14
Distributed by:
14/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
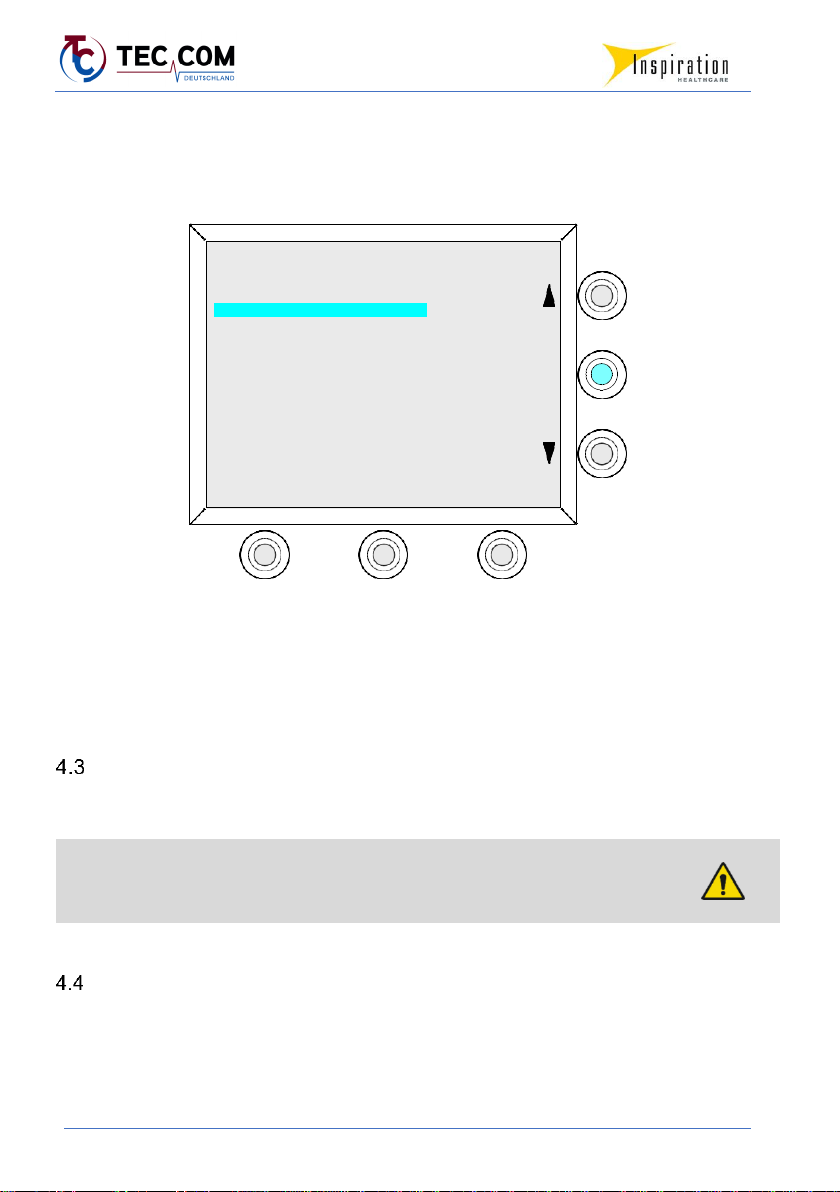
4.2.3 Display Menu
The menu is shown via the display. Individual menu items can be selected using the function
keys and menu arrow keys. The starting point and end point for navigation is always the main
menu. Selected menu items are highlighted in turquoise.
Illustration example, menu language: English
All menu items are accessible to the user except for the SERVICE menu item in the main menu.
This is only accessible with a password for authorized service technicians.
Acoustic alarms are optically supported by symbols on the display. Important instructions, notes
and errors are communicated to the user in turquoise POP-UP windows.
Power cable
The power cable is used to connect the TECOtherm NEO to a protective contact socket for power
supply.
The power cable is only permitted for connection to protective contact sockets
with 100-130 V or 200-240 V and 50-60 Hz. The power cable must have a length
of 2.5 m.
Mattress
The mattress is used for the whole body temperature control of patients, in particular of
newborns and infants. It is connected to the TECOtherm NEO via the hose kit, and temperature
regulation fluid flows through it. To improve the temperature control of the patient, a mattress
can be wrapped around the body as required and closed with straps on the fastener loops.
TECOTHERM NEO Main Menu
Highlight and Select Function Required:
Servo controlled complete treatment mode
Servo control mode (constant rectal temperature)
ConstantMattress Temperature Mode
Alarm check
Display and export of treatment data
Service
Language
Power Off
Ser.-Nr. 2019/13/06
Rev. 063/02.18
Select

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
15/52
Mattresses are available for single and multiple use. Details are listed in Appendix “I APPENDIX
–Equipment and Accessories“. Please feel free to contact our Service Department.
The device with connected mattress must not be filled under pressure. The
mattress must remain flexible and soft so that the patient does not get pressure
marks.
Direct contact of the mattresses with the patient's skin should be avoided,
especially in the case of newborns. A suitable cover cloth is to be used on the
mattress; cf. “I APPENDIX –Equipment and Accessories“.
Example figure: Disposable mattress
Fastener
loops
Coupler plug
Head
section
Fastener
loops

Distributed by:
16/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
Cover cloth (interlayer)
The cover cloth is used for the mutual protection of patients and mattresses.
On the one hand, the patient is protected from contact with temperature regulation fluid in the
event of a leakage from the mattress, and on the other hand, the mattress is protected from
possible contamination by the patient's body. The cover cloths to be used are listed in section
“I APPENDIX –Equipment and Accessories“.
When reusable mattresses are used, the use of a thin, moisture-impermeable
fleece cloth (coated on one side with plastic) is mandatory for the protection
of patients and mattresses.
Hose kit
The hose kit is used for coupling the mattress to the TECOtherm NEO device and consequently
produces the circulation of the temperature regulation fluid.
The hose kit consists of two inner liquid-conducting hoses and the jacket hose for thermal
insulation. The standard length of the hose kit is 2 m. All couplings are self-sealing.
One of the two hoses of the hose kit is marked blue at both ends. The blue markings indicate
which mattress side is connected to which device socket. This facilitates the determination of
the direction of flow between the device and the mattress, since the liquid flows out of the
device to the left hose socket and is returned via the right hose socket.
Coupling connectors
Coupler plug

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
17/52
Refill sets
The TECOtherm NEO device can be filled or refilled with temperature regulation fluid using refill
sets. The refill set consists of a specially prepared 500 ml container with two connecting hoses
and self-sealing coupling plugs.
External temperature sensors
TECOtherm NEO has connections for a rectal temperature sensor and an optional temperature
sensor for measuring skin temperature.
The body core temperature in the rectum can be measured via the rectal temperature sensor
and the temperature regulator can be controlled. The sensor is connected to the device via the
socket marked "R“.
A skin temperature sensor can be used as a reference sensor if required. It is used for
independent temperature monitoring of the patient, but is not necessary for the operation of
the device and is not used for control. It is connected to the device via the socket marked “S”.
Temperature sensors approved by the manufacturer of TECOtherm NEO are listed in the
appendix (cf. I APPENDIX –Equipment and Accessories).
The TECOtherm NEO hypothermic system must only be operated with
temperature sensors approved by the manufacturer.
Observe the correct assignment of the temperature sensors to the connector
sockets R (rectal temperature sensor) and S (skin/skin temperature sensor)
on the TECOtherm NEO device!
Ensure correct positioning of the rectal sensor in the patient! Fix and secure
the sensor against slipping out!

Distributed by:
Version: 21.1 18/06/2019
TECOtherm NEO from software version 063/02.18
19/52
Description of the operating modes
Regardless of the operating mode, all temperatures as well as cooling and warming rates are
logged by the device. These can be read via a USB stick.
5.1.1 Automatic operation by program
The treatment in automatic operation according to programs is planned and defined in advance
by setting temperature-time profiles.
Program 0: In the factory default state of the TECOtherm NEO system, program no. 0 is set,
according to TOBY protocol with the following temperature-time profile:
Rectal in the treatment phase cooling
(therapy phase)
33.5° C
Duration of treatment phase cooling
(therapy phase)
72 h
Rectal temperature after warming
(temperature retention phase)
37° C
Duration of the re-warming phase
7 h
This program cannot be overwritten; it is stored permanently.
The standard values for temperatures and times set in accordance with the TOBY protocol can
be changed by the user within certain limits before the treatment and also during the treatment.
Programs 1–9: Nine programs can be created and saved individually by the user (to be saved as
programs no. 1 to no. 9). The parameters can be defined within the following limits:
Rectal in the treatment phase cooling
(therapy phase)
32 –38° C
Duration of treatment phase cooling
(therapy phase)
1 - 100 h
Rectal temperature after warming
(temperature retention phase)
36 –37° C
Duration of the re-warming phase
1 - 24 h
If changes are made to the specified temperatures or times before the start of the treatment,
the option is provided to store this new set of specifications as a separate treatment program. If
you select this option, this new set of specifications will receive the next available program
number (from 1 to 9) for identification. If the starting point for the changes made was an already
previously created separate treatment program, this can optionally also be redefined instead of
creating an additional treatment program.

Distributed by:
20/52
TECOtherm NEO from software version 063/02.18
Version: 21.1 18/06/2019
After a treatment program has been saved, the user is presented with the option to set this
program as the default. If this option is accepted by pressing “Yes“, this set of specifications are
provided in the future each time this operating mode is selected, so that the treatment can be
started immediately without having to make any changes.
Even after the treatment has been started, the temperature and time specifications can be
changed if necessary by calling the parameter screen with the “Options“ button. However, such
changes cannot be stored in the treatment programs during ongoing treatment. They only apply
to the current treatment.
In operating mode I, there are 4 treatment phases:
1. Phase/cooling phase: In the cooling phase, the temperature is regulated at maximum power
until the target value is reached. The target temperature is preset to 33.5° C in this automated
mode according to the TOBY protocol.
2. Phase/therapy phase: After the setpoint temperature has been reached, the control system
automatically changes to the therapy phase in which the setpoint temperature is maintained for
the duration preselected by the user.
If temperature deviations > 1° C occur during this phase when comparing the measured rectal
temperature with the setpoint temperature, this is detected by the monitoring system and an
alarm is triggered. The device automatically goes into the fallback mode (cf. “5.2 The Fallback
Mode –for Patient Safety“).
3. Phase/warming phase: As soon as the second phase is completed, the warming phase
automatically follows. It is also possible to switch to phase 3 in the “Options“ menu before the
second phase finishes.
The TECOtherm NEO system increases the body core temperature linearly until the selected
target temperature of the temperature retention phase is reached. In case of faults, the control
system reacts in the same way as in phase 2.
4. Phase/temperature retention phase (optional): After completion of re-warming, the
connection of a temperature retention phase is optionally possible. The TECOtherm NEO
maintains a previously defined constant rectal temperature in this phase. You can extend this
phase for any length of time. You can cancel the temperature retention phase by terminating
the program via the menu.
Other manuals for Tecotherm Neo
2
Table of contents
Other Inspiration Medical Equipment manuals