3
The HEMOCHRON Jr. PT test measures the extrinsic coagulation pathway and is sensitive to coagulation factors
VII, X, V, II, and fibrinogen. PT results may be abnormal in patients with liver disease or Vitamin K deficiency.
The test is widely used to monitor oral anticoagulant therapy. The PT test is a unitized test system utilizing a
highly sensitive thromboplastin for improved specificity and sensitivity.
The HEMOCHRON Jr. PT Citrate test performs the same measurement as the PT test, using a citrated whole
blood sample.
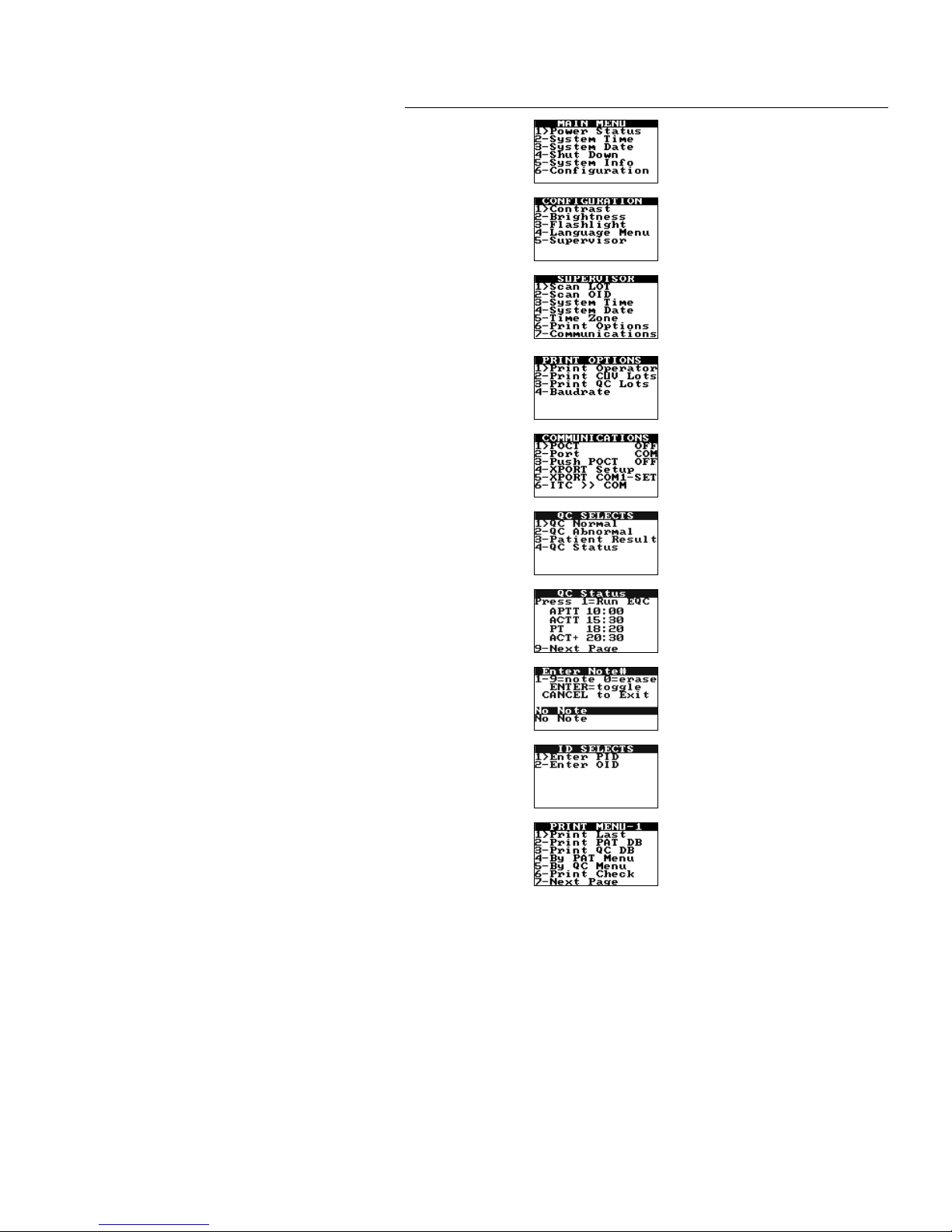
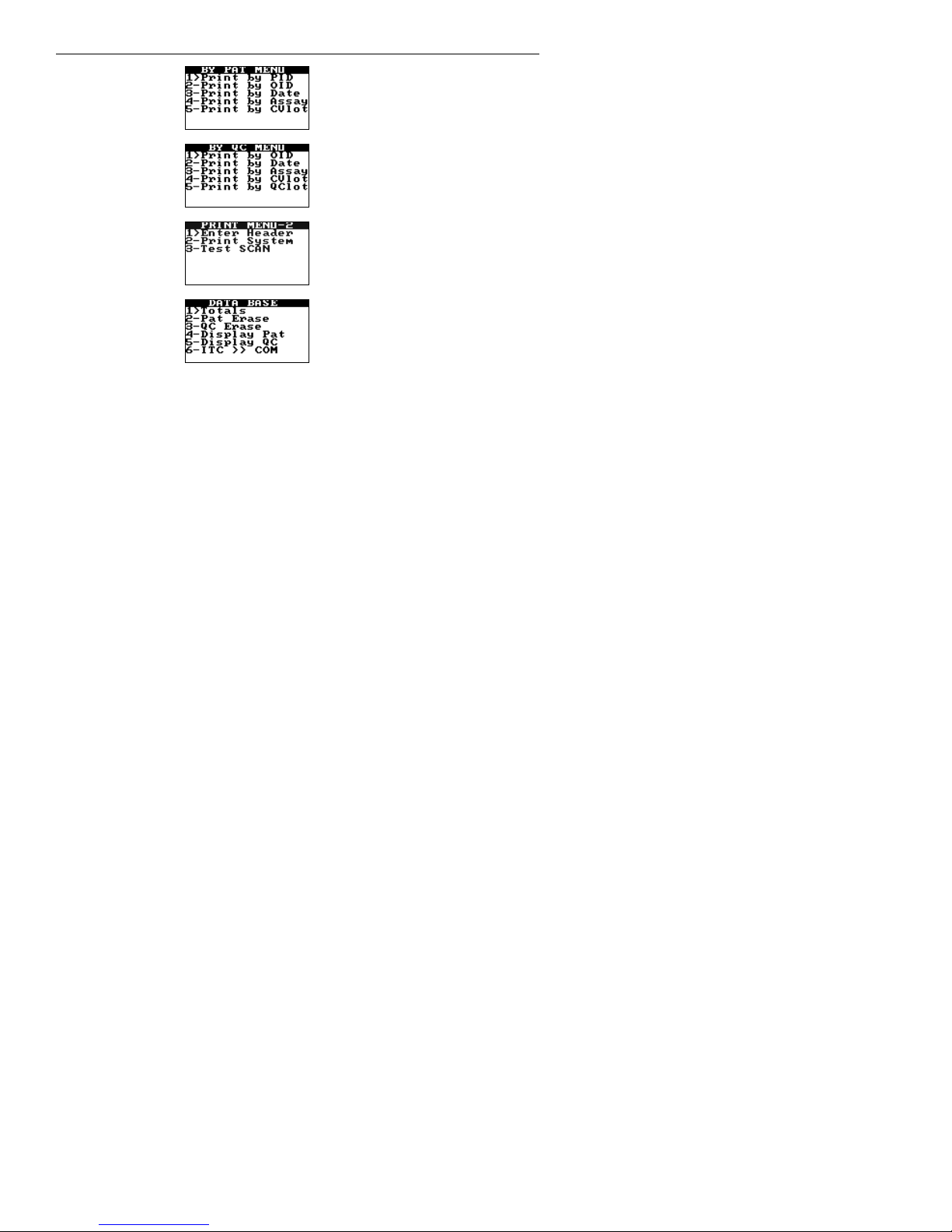
Note: HEMOCHRON Configuration Manager V3.0 or higher is required and is included with the
HEMOCHRON Signature Elite instrument.
PRINCIPLES OF OPERATION
The HEMOCHRON Signature Elite Whole Blood Microcoagulation System provides many features for ease of
use and reliability, including a patented clot detection system, a data storage module, interfaces for a laboratory
computer and/or printer, a streamlined user-interface panel, and an integrated barcode scanner.
The system measures whole blood clotting times using HEMOCHRON Jr. disposable single-use cuvettes. Each
cuvette contains all of the reagents necessary for a specified test.
The operator inserts a cuvette for the test into the instrument and then (if desired) enters information about the
sample or scans the information from a barcode using the integral barcode scanner. After the cuvette has
warmed to 37 °C ±1.0 °C, the instrument beeps, signaling the operator that a blood sample can be added to the
cuvette and the test started.
The operator then places a drop of blood in the sample well of the cuvette and presses the START key. The
instrument measures the required volume of blood and automatically moves it into the cuvette test channel,
where it is mixed with reagents. The remainder of the blood sample, not needed for testing, is automatically
drawn out of the sample well and into an enclosed waste channel on the cuvette.
After mixing with the reagent, the sample is moved back and forth at a predetermined rate within the test
channel and monitored for clot formation. The test channel is maintained at 37 °C ±1.0 °C during the test.
The rate of movement of the sample is monitored by a series of LED optical detectors that are aligned with the
test channel. When the blood clots, the flow of the blood sample within the test channel is impeded, reducing its
rate of flow between the optical detectors.
This reduction in flow below a predetermined value signals to the instrument that a clot has formed. The
instrument also emits an audible beep when clot formation occurs, indicating the end of the test. An internal
timer measures the elapsed time between the start of the test and the clot formation. During the test, the whole
blood clotting time (in seconds) is displayed.
APTT and APTT Citrate results are displayed as plasma equivalent (PE) values, PT and PT Citrate results are
displayed as the International Normalized Ratios (INR) and PE values, and ACT+ and ACT-LR results are
displayed as Celite ACT equivalent time.
The results will remain on the display while the cuvette remains in the instrument.
The result can be automatically printed along with the time and date the test was run, the Patient ID, Operator
ID, and other information, if entered. The result is also saved in an internal database. Up to 600 patient test
results and 600 quality control test results can be stored in the instrument for later printing or downloading.
Individual HEMOCHRON Signature Elite instruments can be customized so that designated quality control tests
must be performed whenever a specified period of time has elapsed and so that information for cuvettes and
liquid controls can be included in test records. The instruments can also be configured so that only authorized
operators can operate the system and/or operators cannot perform certain functions such as deleting test
results from the instrument database. These and other configuration options are entered using HEMOCHRON
Configuration Manager Software on a personal computer.