Ixtur Ltd / MRP-20NK
Technical Specifications
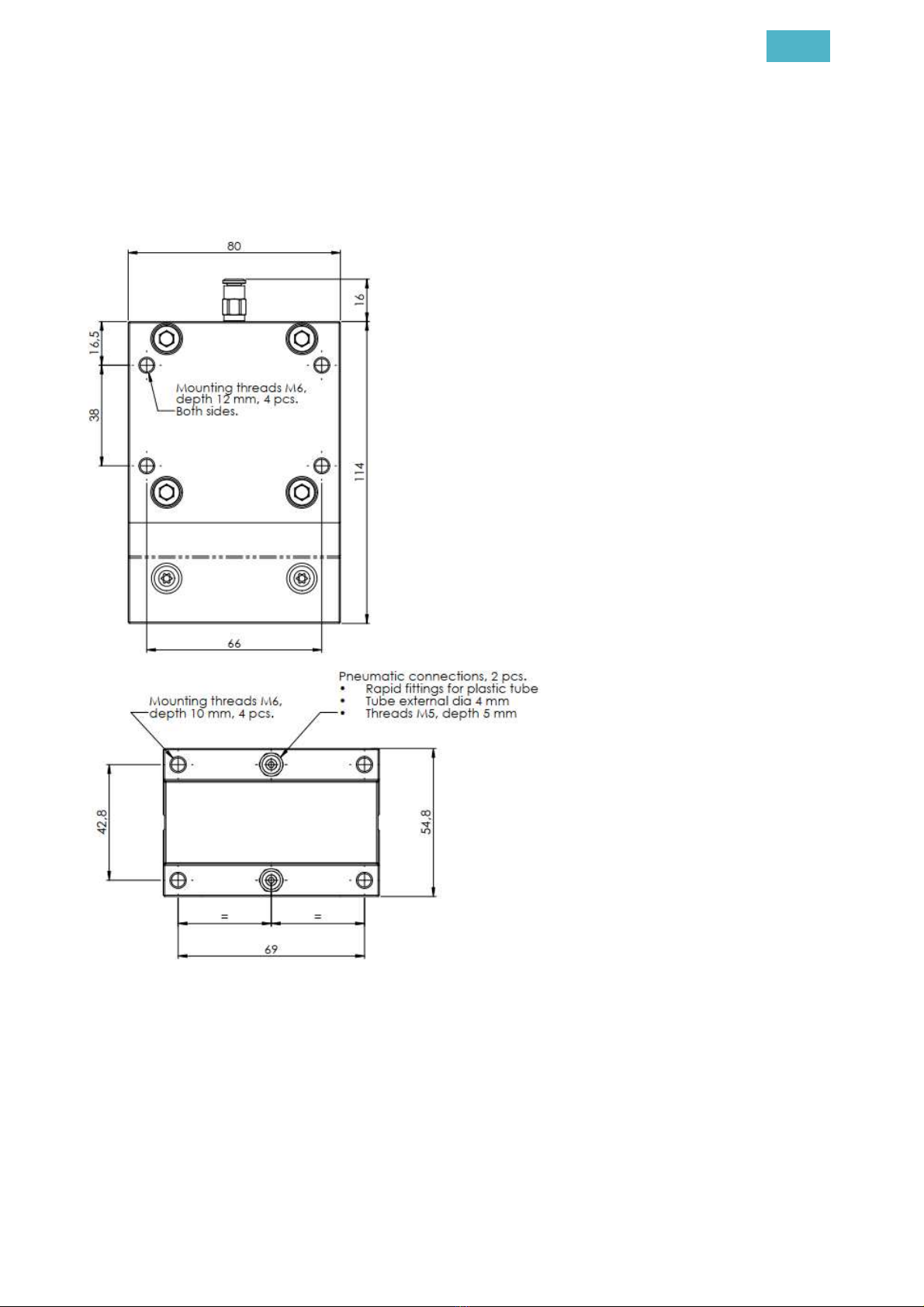
Dimensions:
-Length: 80.0 mm
-Width 54.8 mm
-Height: 114.0 mm
-Weight: 2.4 kg
Capacity:
-Rated holding force: 590 N (S355 S 4 mm plate)
-Rated holding force: 380 N (S355 cylinder ø 14 mm)
-Rated lifting capacity for plate with safety factor 3: 20 kg (S355 S 4 mm)
-Rated lifting capacity for solid round object with safety factor 3: 13 kg (ø 14 mm)
-Residual holding capacity: 0.1 kg
The residual gripping capacity, i.e. the gripping capacity when the magnet is OFF,
varies based on the material and geometry of the gripped part. In extreme cases, parts
up to 0.1 kg may stay attached. The residual capacity is greatest as long as the part
continuously stays in contact with the magnet after the magnet has been turned from
ON to OFF. If the amount of residual capacity is critical to the application, pretesting
with the actual part is recommended.
Environmental conditions:
-Operating temperature: 0 °C … +50 °C
-Storage temperature: -20 °C … +50 °C
-Humidity 0 % … 90 %
-IP67 (The device is protected from dust and temporary immersion up to 1 m)
Requirements for compressed air:
-Recommended pressure: 6.0 bar
-Functional range: 5.0 … 8.0 bar
-Water separation
-Particle filter ≤5µm
Information related to medical implants
-Safety distance for a person with active implanted device is 50 cm of air or other non-
magnetic material. The distance is measured from the magnet or magnetic material
attached to it.
oMaximum static magnetic field of MRP-20NK is 200 mT on the gripping surface.
oInterference with active implanted devices, e.g. cardiac pacemakers – Action level for expo-
sure to static magnetic fields is 0,5 mT [Directive 2013/35/EC].