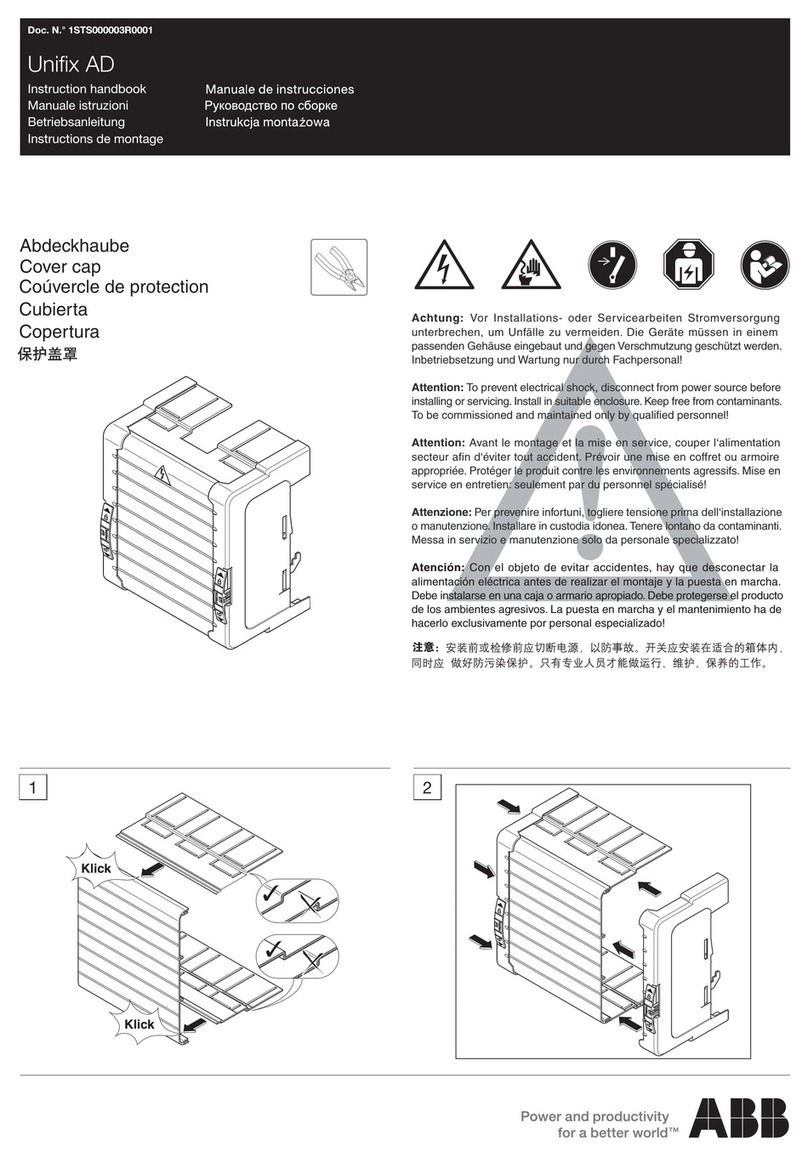
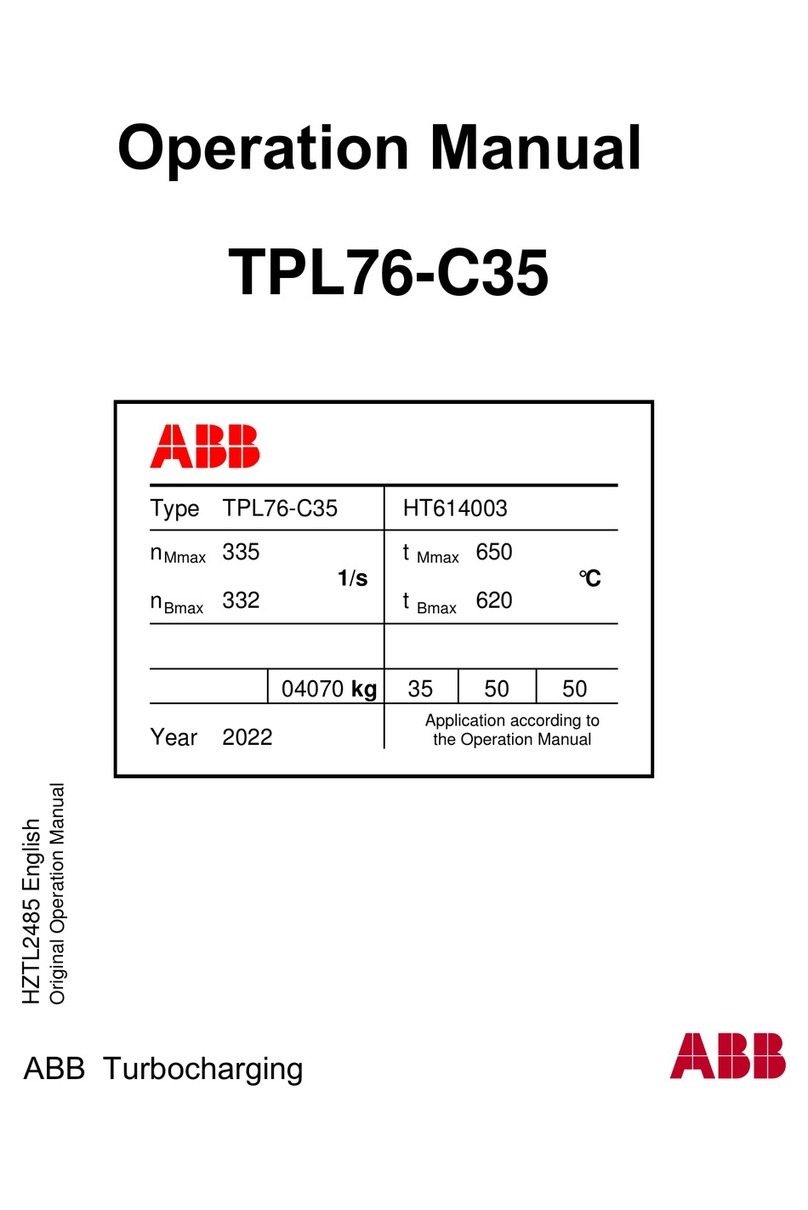
Ixtur Ltd / MRP-31F
Technical Specifications
Dimensions:
- Length: 80.0 mm
- Width 54.8 mm
- Height: 76.0 mm
- Weight: 1.74 kg
Capacity:
- Rated holding force: 910 N (S355 S ≥ 4 mm plate)
- Rated lifting capacity for plate with safety factor 3: 31 kg (S355 S ≥ 4 mm)
- Residual holding capacity: maximum 3 kg
MRP-31F’s magnetic structure (similar to standard lever magnets) makes residual grip-
ping capacity sensitive to material and geometry of the lifted part. In extreme case, a
part up to 3 kg may stay connected to the magnet. This may happen just after the mag-
net has been turned OFF. When this behavior is critical to the application, the actual
parts need to be pretested with the MRP-31F magnet.
Environmental conditions:
- Operating temperature: 0 °C … +50 °C
- Storage temperature: -20 °C … +50 °C
- Humidity 0 % … 90 %
- IP67 (The device is protected from dust and temporary immersion up to 1 m)
Requirements for compressed air:
- Recommended pressure: 6.0 bar
- Functional range: 5.0 … 8.0 bar
- Water separation
- Particle filter ≤ 5µm
Information related to medical implants
- Safety distance for a person with active implanted device is 50 cm of air or other non-mag-
netic material. The distance is measured from the magnet or magnetic material
attached to it.
o Maximum static magnetic field of MRP-31F is 200 mT on the gripping surface.
o Interference with active implanted devices, e.g. cardiac pacemakers – Action level for expo-
sure to static magnetic fields is 0,5 mT [Directive 2013/35/EC].