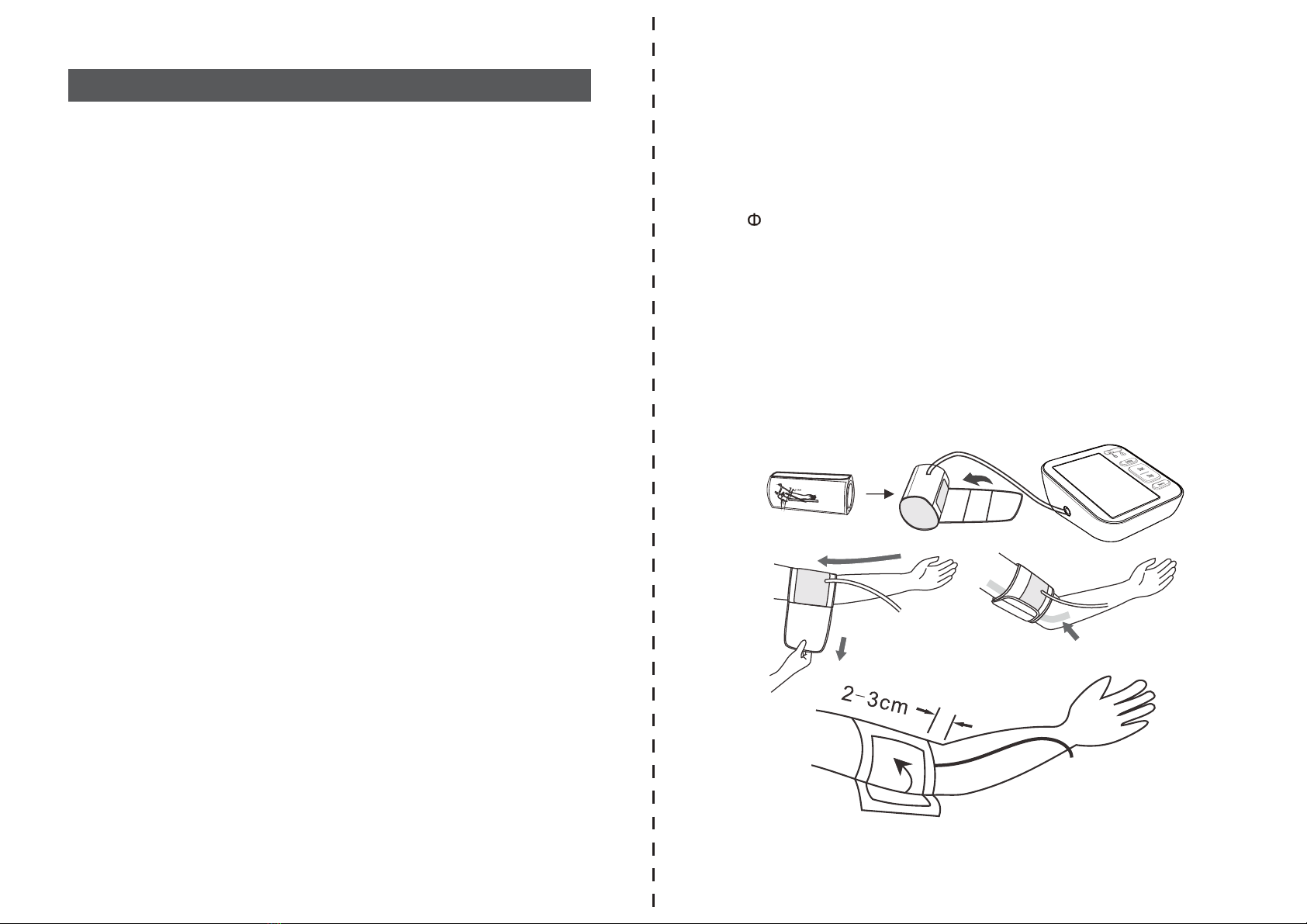
NOTE!
Warning:
or electromagnetic fields in the direct vicinity of the device (e.g. mobile
telephones, microwave ovens) during use. These can lead to erratic results.
occur, refer to local distributor or the manufacturer.
1.Too frequent measurements can cause injury to the PATIENT due to
blood flow interference.
2.Don’t place the cuff over wound part.
3.Pressurization of the CUFF can temporarily cause loss of function of
simultaneously used monitoring ME EQUIPMENT on the same limb.
Warning: The device contains sensitive electronic components. Avoid
strong electrical or electromagnetic fields in the direct vicinity of
the device (e.g. mobile telephones, microwave ovens).These
can lead to temporary impairment of the measuring accuracy.
Warning: Do not use cuffs, AC adapters or batteries other than those
included with this product or replacement parts supplied by the
manufacturer.
Warning: Do not use the batteries and the AC adapter to provide power
at the same time.
Warning: This system may fail to yield specified measurement accuracy if
operated or stored in temperature or humidity conditions outside
the limits stated in the specifications section of this manual.
Warning: The separate ac adapter which is intended to connect USB
interface of Blood Pressure Monitor has not been evaluated
according to IEC 60601-1. The safety of the product shall be
reappraised when it power supply by a separate ac adapter.
Note: To obtain the greatest accuracy from your blood pressure
instrument, it is recommended that the instrument be used within
the specified temperature and the relative humidity, please see
the Technical Specifications.
Note: The cuff is treated as the applied part.The user should contact
the manufacturer for assistance, if needed, in setting up, using
or maintaining the device.
Warning: Remove the battery if the ME EQUIPMENT is not likely to be
used for some time.
Warning: The user must check that the equipment functions safely and
see that it is in proper working condition before being used.
Warning: No modification of this equipment is allowed.
Warning: Use of power adapters
1.Adapter: input 100-240v, 50/60hz output DC 5V 1A
2.Do not to position the device to make it difficult to operate the
disconnection device while using adaptor.
3.Do not be prone to water leakage, high temperature, moisture,
direct sunlight and more or more corrosive gas environment. And
Do not use this product in the above environment.
Warning: The device is not suitable for use in the presence of flammable
anesthetic mixtures with air or with oxygen or nitrous oxide.
Warning: This equipment shall not be serviced or maintained while in use
with the patient.
Warning: The patient is an intended operator, the functions of monitoring
blood pressure and pulse rate can be safely used by patient. The
routine clean and changing batteries can be performed by the
patient.
Caution: To avoid any possibility of accidental strangulation, keep this unit
away from children and do not drape tubing around your neck.
Caution: To avoid damaging the device, keep this unit away from children
and pets.
Caution: The standard material used for the bladder and tubing is latex-free.
Attention: Self-measurement means control, not diagnosis or treatment.
Unusual values must always be discussed with your doctor. Under
no circumstances should you alter the dosages of any drugs
prescribed by your doctor.
Attention: The pulse display is not suitable for checking the frequency of
heart pacemakers!
Attention: In cases of irregular heartbeat, measurements made with this
instrument should only be evaluated after consultation with your
doctor.
Contraindication
Use of this instrument on patients under dialysis therapy or on anticoagulant,
antiplatelets, or steroids could cause internal bleeding.
1.2 Warnings and Precautions