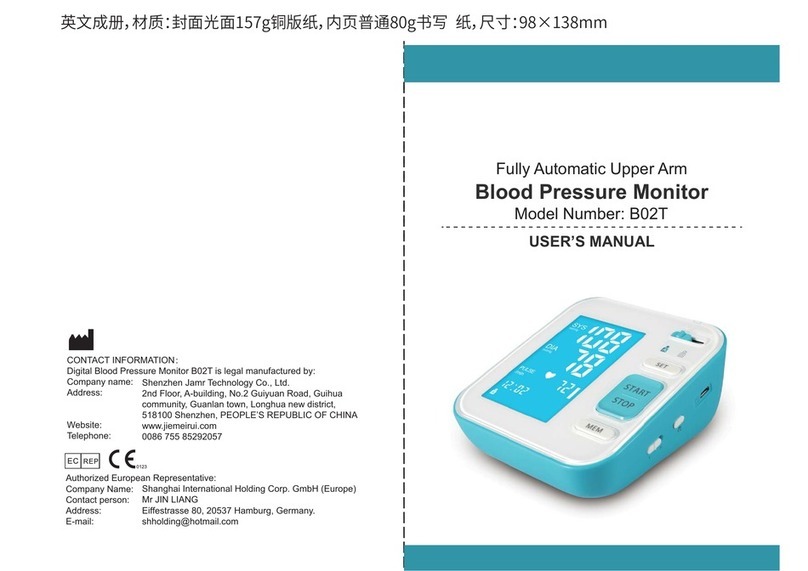
It enables reliable measurement of systolic and diastolic blood pressure as well as pulse
through the oscillometric method.
Before using, please read this instruction manual carefully and then keep it in a safe
place.
• Only a health-care professional is qualified to interpret blood pressure measurements.
• This device is NOT intended to replace regular medical checkups.
• Blood pressure readings obtained by this device should be verified before prescribing or
making adjustments to any medications used to control hypertension. Under no
circumstances should YOU alter the dosages of any drugs prescribed by your physician.
• This monitor is intended for use by adults only. Consult with a physician before using this
instrument on a child.
•In cases of irregular heartbeat , measurements made with this instrument should only be
evaluated after consultation with a physician.
• Host products, including accessories, shall be processed in accordance with local
regulations after reaching the life cycle.
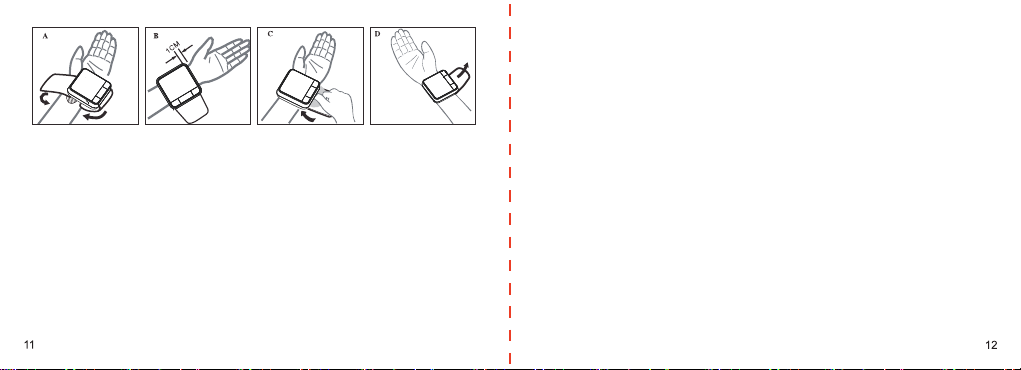
1.Introduction and Intended Use
1.1 Remember…
Warning:
Warning:
Warning:
Warning:
Warning:
Warning:
Warning:
The device contains sensitive electronic components. Avoid strong electrical or
electromagnetic fields in the direct vicinity of the device (e.g. mobile telephones,
microwave ovens).These can lead to temporary impairment of the measuring
accuracy.
The use of other accessories other than those specified or provided by the
equipment manufacturer may cause electromagnetic radiation to increase or
decrease electromagnetic immunity resulting in operational failure.
This system may fail to yield specified measurement accuracy if operated or
stored in temperature or humidity conditions outside the limits stated in the
specifications section of this manual.
Remove the battery if the ME EQUIPMENT is not likely to be used for some time.
The user must check that the equipment functions safely and see that it is in
proper working condition before being used.
No modification of this equipment is allowed.
The device is not suitable for use in the presence of flammable anesthetic
mixtures with air or with oxygen or nitrous oxide.
Warning:
Warning:
This equipment shall not be serviced or maintained while in use with the patient.
The patient is an intended operator, the functions of monitoring blood pressure
and pulse rate can be safely used by patient. The routine clean and changing
batteries can be performed by the patient.
1.2 Warnings and Precautions