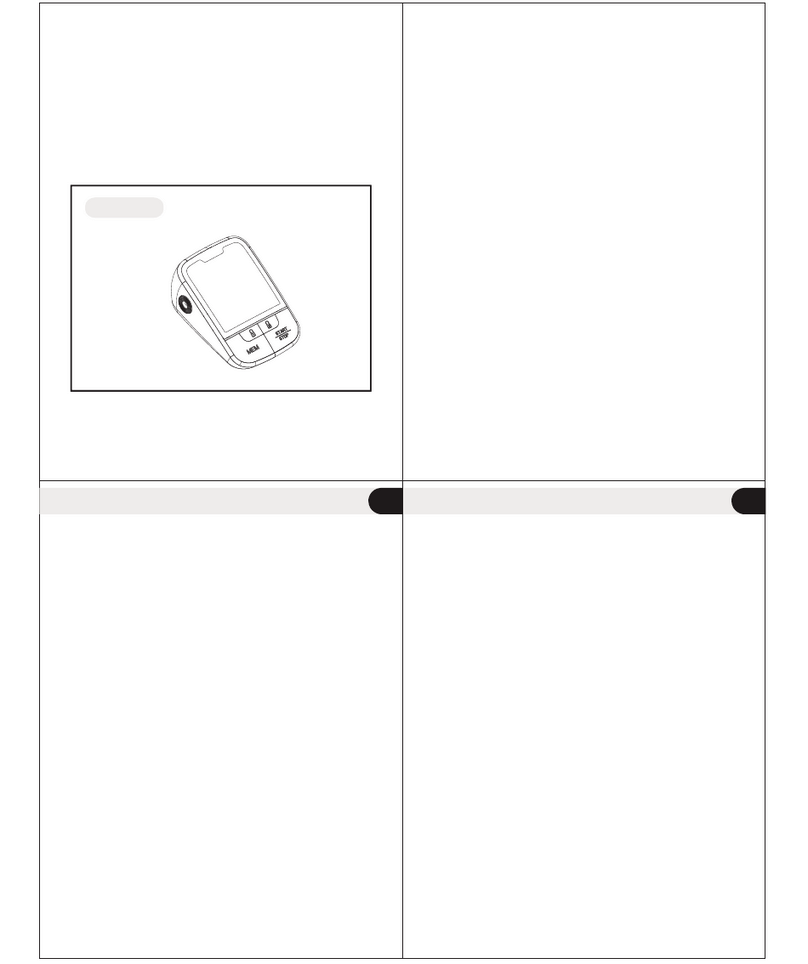
Unit Illustration
Safety Notice Safety Notice
3 4
6
Safety Notice 5
Monitor Unit
Battery cover
AC Adapter Jack
Systolic Blood Pressure
M " " Button
" " Button
"SET" Button
Diastolic Blood Pressure
Pulse Rate
Air Jack
Important Instructions Before Use
1. Do not confuse self-monitoring with self-diagnosis. Blood pressure measurements should
only be interpreted by a health professional who is familiar with your medical history.
2. Contact your physician if test results regularly indicate abnormal readings.
3. If you are taking medication, consult with your physician to determine the most appropriate
time to measure your blood pressure. NEVER change a prescribed medication without first
consulting with your physician.
4. Individuals with serious circulation problems may experience discomfort. Consult your
physician prior to use.
5. For persons with irregular or unstable circulation resulting from diabetes, liver disease,
arteriosclerosis or other medical conditions, there may be variations in blood pressure
values measured at the wrist versus at the upper arm. Monitoring the trends in your blood
pressure taken at either the arm or the wrist is nevertheless useful and important.
6. People suffering from vascular constriction, liver disorders or diabetes, people with
cardiac pacemakers or a weak pulse, and women who are pregnant should consult their
physician before measuring their blood pressure themselves. Different values may be
obtained due to their condition.
7. People suffering from arrhythmias such as atrial or ventricular premature beats or atrial
fibrillation only use this blood pressure monitor in consultation with your doctor. In
certain cases oscillometric measurement method can produce incorrect readings.
15. Product is designed for its intended use only. Do not misuse in any way.
16. Product is not intended for infants or individuals who cannot express their intentions.
17. Prolonged over-inflation of the bladder may cause ecchymoma of your arm.
18. Do not disassemble the unit or arm cuff. Do not attempt to repair.
19. Use only the approved arm cuff for this unit. Use of other arm cuffs may result in
incorrect measurement results.
8.Too frequent measurements can cause injury to the patient due to blood flow interference.
9.The cuff should not be applied over a wound as this can cause further injury.
10.DO NOT attach the cuff to a limb being used for IV infusions or any other intravascular
access, therapy or an arterio-venous (A-V) shunt. The cuff inflation can temporarily
block blood flow, potentially causing harm to the patient.
11.The cuff should not be placed on the arm on the side of a mastectomy. In the case of a
double mastectomy use the side of the least dominant arm.
12.Pressurization of the cuff can temporarily cause loss of function of simultaneously used
monitoring equipment on the same limb.
13.A compressed or kinked connection hose may cause continuous cuff pressure resulting
in blood flow interference and potentially harmful injury to the patient.
14.Check that operation of the unit does not result in prolonged impairment of the circulation
of the patient.
23. Replace batteries when Low Battery Indicator“ ”appears on screen. Replace both
batteries at the same time.
24. Do not mix battery types. Long-life alkaline batteries are recommended.
25. Remove batteries from device when not in operation for more than 3 months.
26. Dispose batteries properly; observe local laws and regulations.
27. Only use a recommended AC adaptor double-insulated complying with EN 60601-1 and
EN 60601-1-2. An unauthorized adapter may cause fire and electric shock.
28. Advising operator that Instruction manual/ Booklet must be consulted.
29. Do not use the device during transport vehicles for influencing measurement accuracy,
such as patient transport in an ambulance or helicopter.
30. Contains small parts that may cause a chocking hazard if swallowed by infants.
31. Please align the polarities of each battery with the +ve and -ve signs
imprinted on the battery housing when you replace the batteries .
32.This ME equipment or ME systems should be only used in shielded location.
33.Use of this equipment adjacent to or stacked with other equipment should be avoided
because it could result in improper operation. If such use is necessary, this equipment and
the other equipment should be observed to verify that they are operating normally.
34.Portable RF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 30 cm (12 inches) to any part of the
unit, including cables specified by the manufacturer. Otherwise, degradation of the
performance of this equipment could result.
20. The system might produce incorrect readings if stored or used outside the manufacturer's
specified temperature and humidity ranges. Make sure to store the blood pressure monitor,
children, pets and pests are outside of accessible range.
21. Do not use the device near strong electrical or electromagnetic fields generated by cell
phones or other devices, they may cause incorrect readings and interference or become
interference source to the device.
22. Do not mix new and old batteries simultaneously.
WARNING SIGNS AND SYMBOLS USED
Keep off Sunlight
Type BF Equipment
Discard the used product to the recycling
collection point according to local
regulations
Instructions For Use MUST be Consulted
Keep Dry
The Bluetooth® Smart word mark and logos are
registered trademarks owned by Bluetooth SIG,
Inc. and any use of such marks by JOYTECH
Healthcare Co.,Ltd.
Federla Commulcation Commission (FCC) Interference Statement
1.This device complies with part 15 of the FCC Rules. Operation is subject to the condition
that this device does not cause harmful interference.
2.This device is verified to comply with part 15 of the FCC Rules for use with cable television
service.
3.This device complies with part 15 of the FCC Rules. Operation is subject to the following two
conditions:
(1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that may cause
undesired operation. Please note that changes or modifications not expressly approved by the
party responsible for compliance could void the user's authority to operate the equipment.
4.This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment generates,
uses and can radiate radio frequency energy and, if not installed and used in accordance with the
instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one or more
of the following measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which the receiver
is connected.
—Consult the dealer or an experienced radio/TV technician for help.