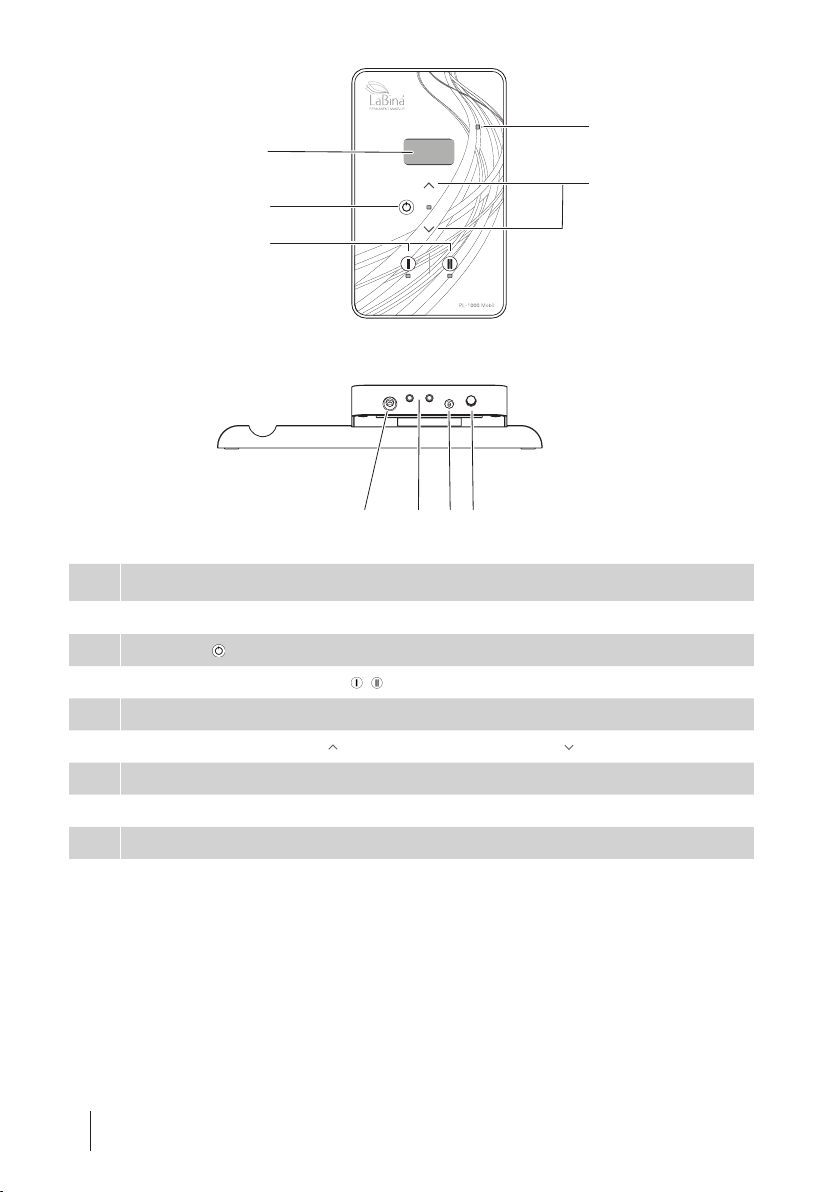
8EN PL-1000 Mobil
EAvoid sources of interference by ensuring that portable or mobile radio devices are not
operated in the vicinity of the device.
EWhen being handled, protect the handpiece and the handpiece cable from contamination
by bodily fluids or substances contaminated with bodily fluids by means of a handpiece
cover (see chapter "5.6 Inserting or replacing the needle cartridge" on page 24).
EIf the device is not being used, disconnect it from the power supply and place the hand-
piece in the handpiece tray so that it cannot roll away or fall.
EObserve the technical data provided in this operating manual and comply with the oper-
ating, transport and storage conditions (see chapter "8 Transport and storage condi-
tions" on page 32).
ESend the device to a specialist retailer for inspection if there are signs of damage, if it
does not function as normal or if liquids enter the device or handpiece.
EIt is recommended that the device is sent to a specialist retailer for inspection at regular
24 month intervals. In doing so, refer to chapter "7.1 Inspection" on page 30.
Important health and safety precautions
Please observe the following instructions in order to prevent contaminations or infectious
diseases being transmitted to the customers or users during treatment:
EFollow all of the steps for disinfecting the equipment before treatment (see chapter
"5.5 Cleaning and disinfecting the equipment" on page 23).
EUse disposable gloves made from nitrile or latex during the treatment and disinfect these
before use. Observe the applicable directives for your country when selecting suitable
disinfectants.
EClean the respective area of the customer's skin with a mild cleaning agent and disin-
fectant before treatment. Observe the applicable directives for your country when select-
ing suitable disinfectants.
EFor the treatment, use only micropigmentation inks that have been tested and approved
for insertion into human skin. Observe the applicable directives for your country when se-
lecting these. If micropigmentation inks that are not intended for permanent make-up
treatment or whose sterility is not guaranteed, are introduced into the skin, this can result
in infections or possible side effects.
EUse only new, sterile packaged needle cartridges for each customer. Before treatment
ensure that the packaging is undamaged and the expiry date has not passed. Note the
batch number of the needle cartridge used in the corresponding customer file in order to
be able to pass this on to the manufacturer in the event of any problems.
Eneedle cartridges are sterile disposable products (consumables) and are only ever per-
mitted to be used once!
EDispose of used or faulty needle cartridges as well as needle cartridges whose packag-
ing is damaged in a non-penetrable container (sharps container) in accordance with the
regulations applicable in your country.
2.3