Page 10
The following list represents patient populations for whom use of
the ACT monitoring system is most appropriate. This list should be
used in conjunction with Medicare and other payor medical
necessity guidelines:
Patients with dizziness or lightheadedness
Patients with palpitations
Patients with syncope of unknown etiology
Patients who require monitoring for non-life-threatening
arrhythmias, such as Atrial Fibrillation, Supra-ventricular
Arrhythmias, evaluation of various Brady arrhythmias. This
includes post-operative monitoring for these rhythms.
Patients recovering from coronary artery bypass graft (CABG)
surgery who require monitoring for arrhythmias
Patients requiring monitoring for arrhythmias-including co-
morbid conditions such as hyperthyroidism or chronic lung
disease
Patients with obstructive sleep apnea to evaluate possible
nocturnal arrhythmias
Patients requiring arrhythmia evaluation for etiology of stroke
or transient cerebral ischemia, possibly secondary to Atrial
Fibrillation
To use the ACT monitoring system, the user or primary care
provider must be able to perform all of the following:
Understand the principle of operation and system messages
described in this manual
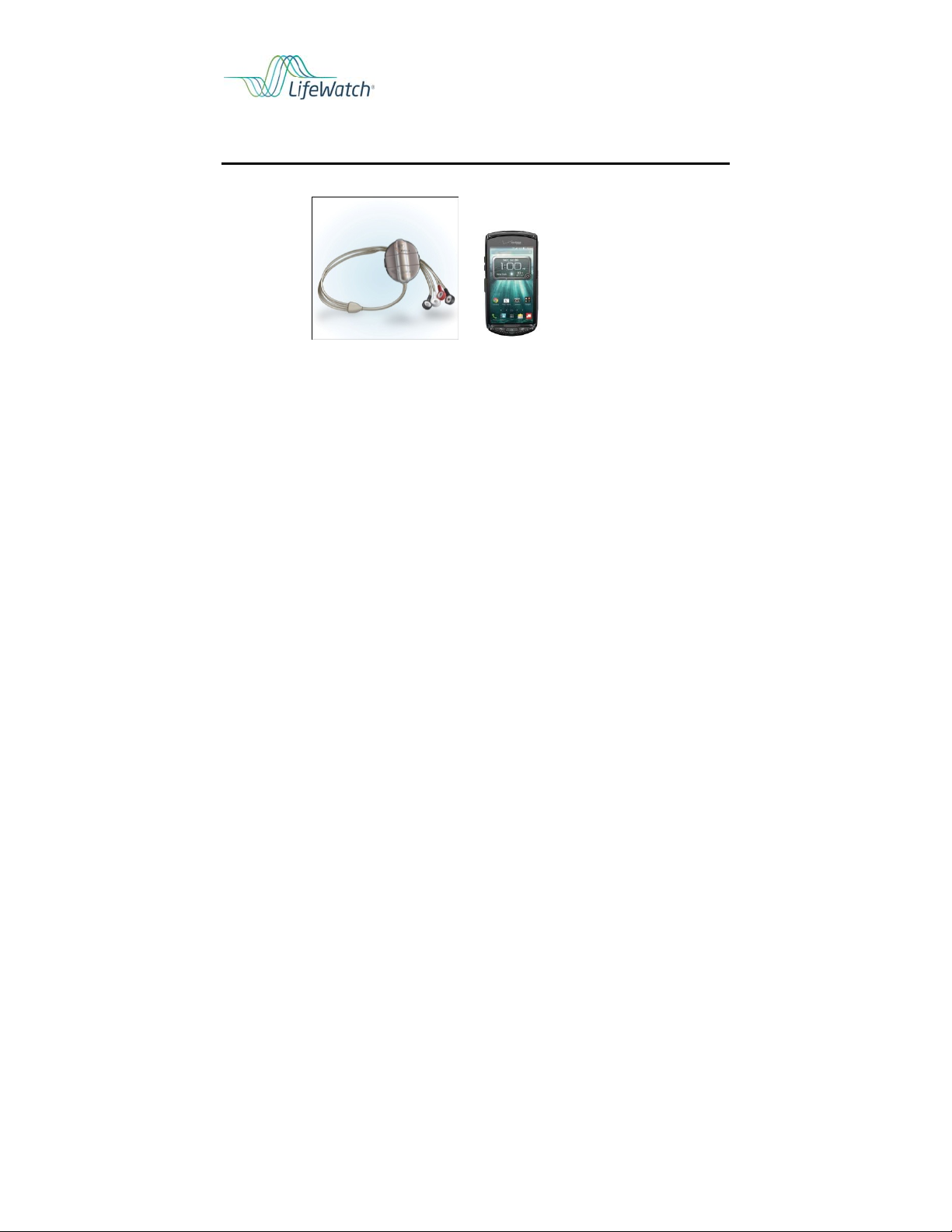
Place the sensor and electrodes on the chest
Operate a handheld device (cellular phone)
Keep monitor nearby the user at all times
The ACT monitoring system is safe for use by patients wearing an
oxygen mask for breathing. See Warnings for other uses in
oxygen rich environments.
The ACT monitoring system is not water resistant and must not
get wet. Do not use or store the ACT monitoring system where
liquids of any nature may come into contact with it. Raindrops,