18 19
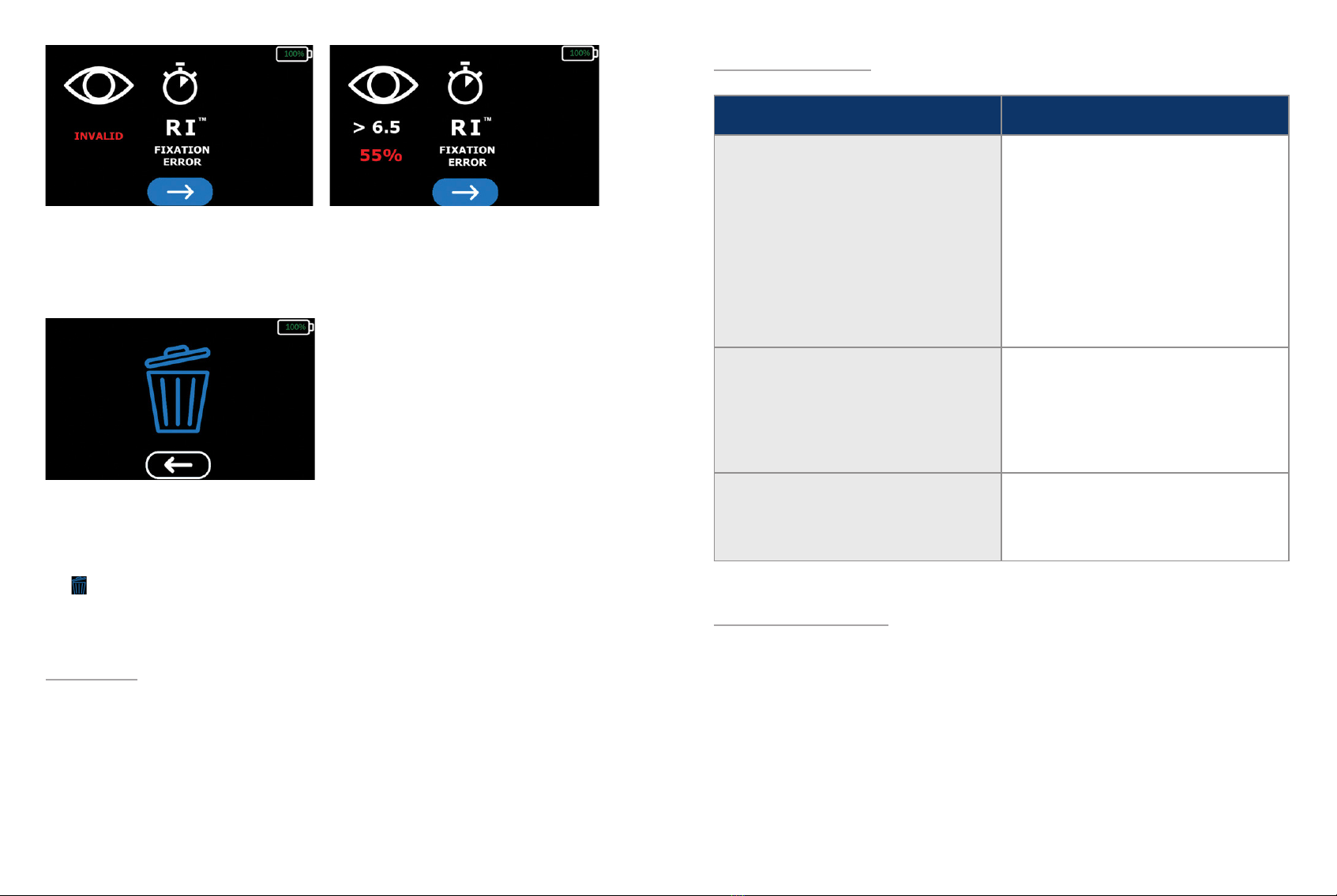
A warning will be generated if a bleaching error (INVALID) was detected or if the xation error is above
30%. A bleaching error or xation rate higher than 30% indicate the results are not valid and the patient
should be retested. After photobleaching, the test eye should not be retested for at least 30 minutes to
ensure a reliable test result.
The system will pause here until the technician has recorded the test results. If the system is powered
down while on this screen, it will automatically display the results the next time the system is booted. To
proceed to the conrmation screen, select the blue button with the white arrow at the bottom of the
screen. To return to the results screen, select the blue button with the white arrow under the “delete”
icon . To conrm the results have been successfully recorded, navigate to and select the “delete” icon.
The results must be deleted before proceeding to another test.
7. Cleaning
Never immerse the AdaptDx Pro in water or other uids, spray, pour or spill liquid onto the AdaptDx
Pro, its accessories, connections, or openings in the device. Dry any liquid on the surface of the device
immediately.
Required cleaning includes regular swabbing of the patient contact points and removal of debris from view
windows, plus periodic cleaning of the display screen. The patient contact points (rubber eyecups, padded
liner, adjustable headstrap, handheld controller) should be wiped between each patient. The use of isopropyl
wipes is recommended. View windows should be gently cleaned with a lens cloth and/or compressed air.
8. Troubleshooting
DESCRIPTION ACTIONS AND/OR SOLUTIONS
Unrecoverable Error (UE) Screen The user should call technical support using
the number on the screen and report UE
Code and Serial Number of the device to the
technical support representative for guidance.
The device will automatically power down
after 1 minute. To restart the device, press and
hold the power button until the power button
illuminates with a green light. The device will
return to the home screen.
Frozen Screen Power down the device by holding down
the power button until the green light on the
power button turns off. To restart the device,
press and hold the power button until the
power button illuminates with a green light.
Abort Test Press and hold the power button for 2
seconds. The device will abort the test and
return to the home screen.
9. Customer Support
For technical support or to order supplies and replacement parts, contact the device supplier. User
manuals are available for download in other languages at maculogix.com/manuals