novocure Optune NovoTTF 200A User manual

USER MANUAL
Document number QSD-EUUM-002-EN
Version 02

3
TABLE OF CONTENTS
1
About Optune®Treatment Kit............................................................. 5
2
Indications for Use ........................................................................... 6
3
Contraindications, Warnings & Precautions ........................................... 7
4
What are the Risks of Treatment with Optune Treatment Kit? ................ 11
5
What are the Benefits of Treatment with Optune Treatment Kit? ............ 11
6
Overview of Optune Treatment Kit .................................................... 13
7
The Device.................................................................................... 14
8
Before You Begin............................................................................ 15
9
Removing the INE Transducer Array from Its Package .......................... 16
10
Preparing Your Head for INE Transducer Array Placement ..................... 17
11
Placing The INE Transducer Arrays On Your Head ................................ 18
12
Connecting the INE Transducer Arrays to the Device............................ 20
13
Starting & Stopping the Device......................................................... 21
14
Connecting & Disconnecting the Battery............................................. 18
15
Charging the Battery ...................................................................... 22
16
Using the Power Supply................................................................... 24
17
The Connection Cable & Box ............................................................ 26
18
Carrying the Device ........................................................................ 29
19
Glossary of Graphic Symbols ............................................................ 30
20
Disposal........................................................................................ 34
21
Environmental Conditions for Normal Operation, Storage and Transportation
34
22
Troubleshooting ............................................................................. 36
23
Assistance & Information.................................................................42
24
Appendix A –Applicable Standards ................................................... 43
25
Appendix B –Input Output Specifications........................................... 46
26
Appendix C –Emitted Radiation & Electromagnetic Compatibility ........... 47
27
Appendix D –Glossary .................................................................... 54
28
Appendix E –Expected Service Life ................................................... 55

4
This manual is intended for patients receiving TTFieldstreatment using the Optune®
Treatment Kit and INE Transducer arrays (Sterile).

5
1
About Optune®Treatment Kit
Optune Treatment Kit a portable medical device. It delivers electric fields called
“TTFields” to the tumor in the brain using INE Transducer arrays. TTFields are intended
to kill cancer cells. The TTFields are transmitted at a frequency of 200 KHz and up to
707mA RMS output current.
Your doctor has prescribed Optune Treatment Kit for use at home. You may be able to
use Optune Treatment Kit on your own, or you may need help from a doctor, family
member, or other caregiver. Use Optune Treatment Kit as many hours per day as
possible, at least 18 hours per day. Only take short breaks for personal needs.
Optune Treatment Kit is portable and has the ability to run on batteries. You can
continue your normal daily life while carrying the device in a shoulder bag or backpack.
The Treatment Kit includes four rechargeable batteries. Each battery will last for up to
two or three hours. For sleeping, or other times when you plan to stay in the same
place for a while, plug the device power supply into a standard wall outlet.
Optune does not need regular maintenance. The Optune Treatment Kit also does not
have any settings for you to change.
The only things you need to do are check that the device has a power source connected
(a charged battery plugged into the device, or is connected to a power supply plugged
into the wall) and turn it on and off. If the device is not working, an audible error
indicator will beep.
A simple Troubleshooting Guide is provided in this manual (Section 21). You can also
call the 24-hour technical support telephone number (Section 22).
Shave your scalp and change the INE Transducer arrays twice a week. Keep periods of
time off from treatment to a minimum.
Interrupt treatment only for personal needs such as bathing, exercise, or any time
where the device may be a distraction. Stop treatment to replace the INE Transducer
arrays.
To take a shower, unplug the INE Transducer arrays from the device (leave the INE
Transducer arrays on your head) and put a shower cap on your head so they do not get
wet. You can take a full shower and wet your head when you are not wearing the INE
Transducer arrays (for example, when you have taken them off but before replacing
them with a newpair). You can wear a wig or hat over the INE Transducer arrays, if
you wish.

6
2
Indications for Use
Optune Treatment Kit is intended for the treatment of patients with newly diagnosed
GBM and for the treatment of patients with recurrent GBM.
Newly diagnosed GBM
NovoTTF-200A (OptuneTM) Treatment Kit is intended for the treatment of patients with
newly diagnosed GBM, after surgery and radiotherapy with adjuvant Temozolomide,
concomitant to maintenance Temozolomide. The treatment is intended for adult
patients, 18 years of age or older, and should be started more than 4 weeks after
surgery and radiation therapy with adjuvant Temozolomide. Treatment may be given
together with maintenance Temozolomide (according to the prescribing information in
the Temozolomide package insert) and after maintenance Temozolomide is stopped.
Recurrent GBM
NovoTTF-200A (OptuneTM) Treatment Kit is intended for the treatment of patients with
recurrent GBM who have progressed after surgery, radiotherapy and Temozolomide
treatment for their primary disease. The treatment is intended for adult patients, 18
years of age or older, and should be started more than 4 weeks after the latest
surgery, radiation therapy or chemotherapy.

7
3
Contraindications, Warnings & Precautions
CONTRAINDICATIONS
Do not use Optune Treatment Kit if you are pregnant, think you might be pregnant, or
are trying to get pregnant. If you are a woman who is able to get pregnant, you must
use birth control when using the device. Optune Treatment Kit was not tested in
pregnant women.
Do not use Optune Treatment Kit if you have significant additional neurological
disease (primary seizure disorder, dementia, Progressive degenerative neurological
disorder, Meningitis or encephalitis, Hydrocephalus associated with increased
intracranial pressure)
Do not use Optune Treatment Kit if you are known to be sensitive to conductive
hydrogels like the gel used on electrocardiogram (ECG) stickers or transcutaneous
electrical nerve stimulation (TENS) electrodes. In this case, skin contact with the gel
used with Optune Treatment Kit may commonly cause increased redness and itching,
and rarely may even lead to severe allergic reactions such as shock and respiratory
failure.
Do not use Optune if you have an active implanted medical device, a skull defect
(such as, missing bone with no replacement) or bullet fragments. Examples of active
electronic devices include deep brain stimulators, spinal cord stimulators, vagus nerve
stimulators, pacemakers and defibrillators. Use of Optune together with implanted
electronic devices has not been tested and may lead to malfunctioning of the
implanted device. Use of Optune together with skull defects or bullet fragments has
not been tested and may possibly lead to tissue damage or render Optune ineffective.

8
WARNINGS
Warning - Use Optune Treatment Kit only after receiving training from qualified
personnel, such as your doctor, a nurse, other medical personnel, or Novocure Device
Support Specialist who have completed a training course given by the device
manufacturer (Novocure). Your training will include a detailed review of this manual
and practice in the use of the system. In addition, you will be trained in what to do if
there are problems with treatment. Use of Optune Treatment Kit without receiving this
training can result in breaks in treatment and may rarely cause increased scalp rash,
open sores on your head, allergic reactions or even an electric shock.
Warning - Do not use Optune Treatment Kit if you are younger than 18 years of age.
It is unknown what side effects the device may cause in these cases or if it will be
effective.
Warning - In case of skin irritation, which appears as redness under the transducer
arrays (a mild rash), talk to your physician before starting any treatment for skin
irritation. Your physician may recommend using use over-the-counter topical steroids
when replacing transducer arrays. This will help relieve your skin irritation. If you do
not use this cream, the skin irritation can become more serious and may even lead to
skin break down, infections, pain and blisters. If this happens, stop using the topical
steroid cream and contact your doctor. Your doctor will supply you with an antibiotic
cream to use when replacing transducer arrays. If you do not use this cream, your
symptoms may continue and your doctor may ask you to take a break from treatment
until your skin heals. Taking a break from treatment may lower your chance to
respond to treatment.
Warning - All servicing procedures must be performed by qualified and trained
personnel. If you attempt to open and service the system alone you may cause
damage to the system. You could also get an electric shock by touching the inner
parts of the device.
Warning -No modification of this equipment is allowed.
Warning – re-use of INE Transducer arrays can lead to poor contact with the scalp and
may cause the device to alarm and stop working. Re-use of INE Transducer arrays can
lead to worsening of the skin inflammation and rarely even to local infection. If you
suffer from an infection on your scalp (puss, swelling and warmth) consult with your
physician immediately.

9
PRECAUTIONS
Caution -Keep the Optune Treatment Kit out of the reach of children and pets.
Caution -Do not use any parts that do not come with the Optune Treatment Kit or
that were not sent to you by the device manufacturer or given to you by your doctor.
Caution -Do not use the Optune Treatment Kit if any parts look damaged (torn wires,
loose connectors, loose sockets, cracks or breaks in the plastic case).
Caution -Do not wet the device or INE Transducer arrays. Getting the device wet may
damage it, preventing you from receiving treatment for the right amount of time.
Getting the INE Transducer arrays very wet is likely to cause the INE Transducer
arrays to come loose from your head. If this happens, the device will operate the
notification signal and you will need to change the INE Transducer arrays.
Caution -Before connecting or disconnecting the INE Transducer arrays, make sure
that the Optune power switch is in the OFF position. Disconnecting INE Transducer
arrays when the device is running will cause a device notification signal to go off, and
could damage the device.
Caution -Connection Cable may pose a hazard of strangulation. Avoid wearing the
connection cable around your neck.
Caution –there is a hazard of falling due to entanglement in the connection cable.
You may consider clipping the cable to your belt .

10
NOTICES
Notice! Optune Treatment Kit is to be used with INE transducer arrays only.
Notice! Optune Treatment Kit and INE Transducer arrays will activate metal detectors.
Notice! You should use the Optune Treatment Kit for at least 18 hours a day to get
the best response to treatment. Using Optune Treatment Kit for less than 18 hours a
day lowers the chances that you will respond to treatment.
Notice! Do not stop using the Optune Treatment Kit even if you have used it less than
the recommended 18 hours per day. You should stop using the device only if your
doctor tells you to. Stopping treatment could lower the chances that you will respond
to treatment.
Notice! If you plan to be away from home for more than 2 hours, carry an extra
battery and/or the power supply with you in case the battery you are using runs out.
If you do not take a spare battery and/or the power supply you may have a break in
your treatment. Breaks in treatment may lower your chance to respond to treatment.
Notice! Batteries may weaken over time and need to be replaced. You will know this
has happened when the amount of time the device can run on a fully charged battery
begins to shorten. For example, if the low battery indicator lights up within only 1.5
hours from the start of treatment, replace the battery. If you do not have
replacement batteries when your batteries run out, you may have a break in your
treatment. Breaks in treatment may lower your chance to respond to treatment.
Notice! Do not block the device vents located on the front and bottom of the Optune
device. Blocking the vents may cause the device to overheat and operate the
notification signal, leading to a break in treatment. If this happens, unblock the vents,
wait 5 minutes and restart the device.
Notice! Do not block the battery charger vents located on the sides of the battery
chargers. Blocking the vents may cause the charger to overheat. This could prevent
your batteries from charging.

11
4
What are the Risks of Treatment with Optune
Treatment Kit?
Skin irritation is often seen under the INE Transducer arrays when using the Optune
Treatment Kit.This will look like a red rash, small sores or blisters on your scalp. In
general, Optune will not cause skin damage that cannot be fixed. The irritation can
be treated with topical steroid cream or by moving the INE Transducer arrays. If you
do not use the topical steroid cream, the skin irritation could become more serious.
This may lead to open sores, infections, pain and blisters. If this happens, stop using
the steroid cream and contact your doctor.
5
What are the Benefits of Treatment with Optune
Treatment Kit?
Patients using Optune after their tumor reappeared lived a similar amount of time
compared to patients using cancer drugs. In the clinical study, half of the patients in
both groups lived for more than 6.4 months. 22 out of each 100 patients lived for
one year or longer.
Patients using Optune after their tumor reappeared had a better quality of life
On the following page is a table showing the effects on the benefit of the device,
when it is used correctly or incorrectly after the tumor reappeared.
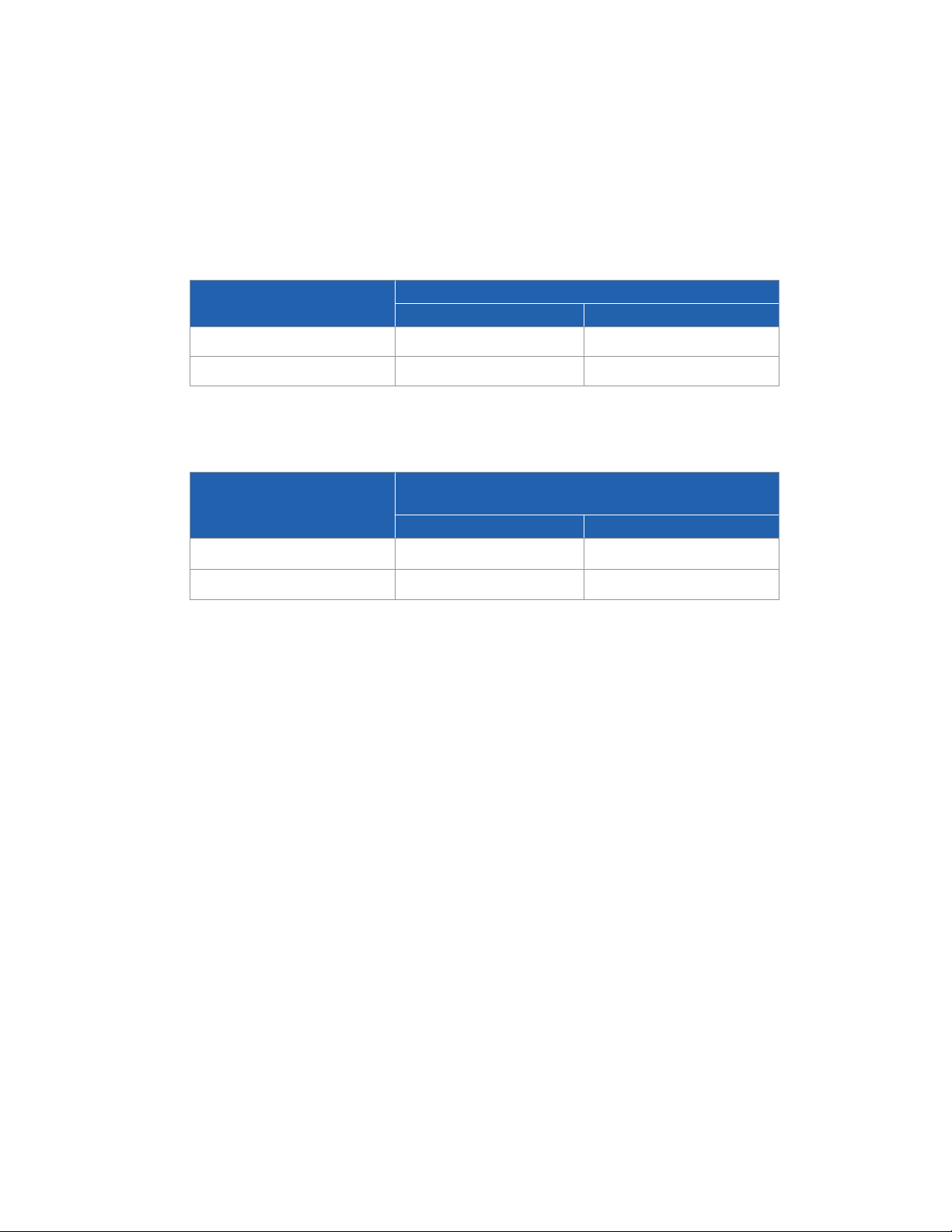
Benefit from Correct and Incorrect Use of Optune
Event
Likelihood of
Event
Outcome Likelihood of
Outcome
Correct use
Use of the device
for at least 18 hours
a day
85 out of 98
subjects (87%)
Survival 3 months
longer compared to
subjects treated
less than 18 hours
a day
81 out of 85 (95%)
Incorrect use
Use of the
device for less
than 18 hours
a day
13 out of 98
subjects (13%)
Survival 3 months
shorter compared
to subjects treated
at least 18 hours a
day
12 out of 13 (92%)
Wetting the device
or soaking the
transducer arrays
Unknown
Treatment break
Unknown
Handling of the
device by
children
Unknown
Treatment break
Unknown
12
In the clinical study using Optune with temozolomide before patients’ tumors
reappeared, the time from the start of treatment to death was measured when half of
the patients had joined the study as well as at the time when all of the total 700 patients
had joined the study. The table below shows the amount of time that patients who used
Optune with temozolomide were observed to be alive longer than patients who used
temozolomide alone.
Benefit of Optune + Temozolomide
Half of Patients in Study
All Patients in Study
Correct use Almost 5 months longer Almost 7 months longer
All subjects 3 months longer Almost 5 months longer
In addition, more patients who used Optune with temozolomide were alive after 2 years
than patients using temozolomide alone
Patients Alive 2 Years after the Start of Treatment
(Optune + Temozolomide vs. Temozolomide Alone)
Half of Patients in Study
All Patients in Study
Correct use
48% vs. 32% 43% vs. 25%
All subjects
48% vs. 34% 43% vs. 31%
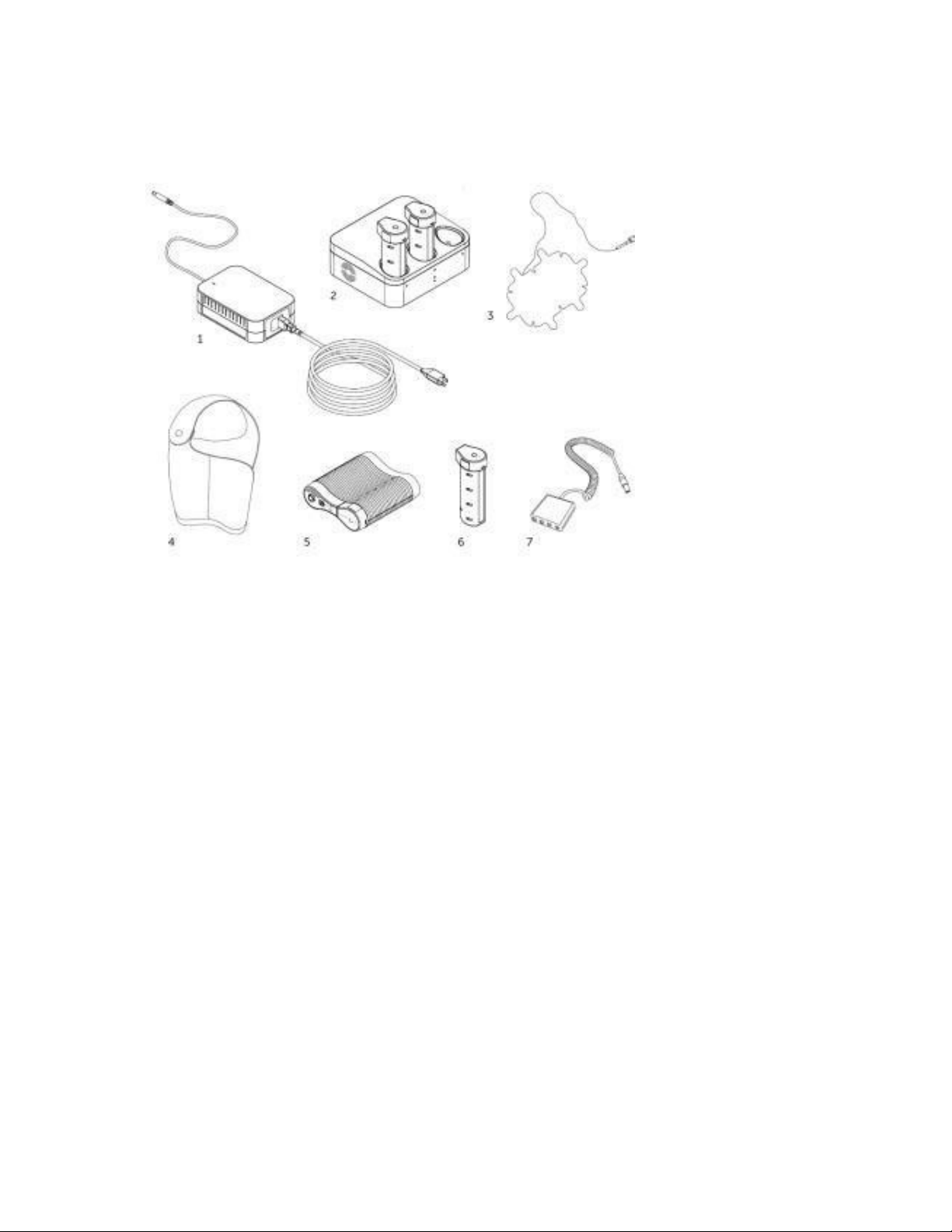
13
6
Overview of Optune Treatment Kit
1
Plug in power supply (Model SPS9100)
2
Charger for batteries (Model ICH9100)
3
Insulated Transducer array (INE) –(Model INE9020 and INE9020W)
4
Device & battery carrying bag (Model BAG9100)
5
Optune electric field generator (the Device) (Model TFH9100)
6
Battery (Model IBH9100)
7
Connection cable & box (Model CAD9100)

14
7
The Device
The Optune Treatment Kit treatment parameters are preset and cannot be changed by
the patient. TTField treatment should be kept on as continuously as possible (24 hours
a day, 7 days a week). Although 100% treatment time is impossible, breaks from
treatment should be kept as short as possible.
You will need to learn how to place it in a carrying bag, connect a battery and operate
the system.
The following controls will allow you to operate the Optune device:
1 Optune power button 2 Power Supply Connection cable socket 3 TTField therapy ON/OFF button
4 Power ON/ Error / Low Battery indicator 5 Connection Cable (CAD) socket 6 Battery Gauge
Note: Instruction on how to use INE Transducer arrays can be found in the INE
Transducer array User Manual supplied with the INE Transducer arrays.

15
8
Before You Begin
You will need to use four (4) INE Transducer arrays at one time. Change these 4 INE
Transducer arrays twice a week to continue treatment with Optune® Treatment Kit.
You may change the INE Transducer arrays with the help of a doctor, a nurse or
caregiver if needed.
Make sure you have an adequate supply of INE Transducer arrays to keep you going
until your next visit to your physician.
Before using an INE Transducer array make sure its package is sealed. Do not use an
INE Transducer array which has been opened previously.
Although the transducer arrays are provided in indivi dual sterile packages to minimize
infection risk, you and/or your caregiver can take additional steps to further reduce
the risk of infection: Always wash your hands prior to application and removal of
transducer arrays; Wash your scalp between transducer array exchanges; Clean the
electric razor per manufacturer’s guidelines after every shave.

16
9
Removing the INE Transducer Array from Its
Package
Wash your hands before opening the envelope with the INE Transducer Arrays.
Open the see through envelope of four (4) INE Transducer arrays by gently pulling
apart the opposing edges of the envelope as shown in the illustration.

17
10
Preparing Your Head for INE Transducer Array
Placement
Wash your head with a gentle shampoo.
If this is the first time you have used the INE Transducer arrays, ignore this step and
skip ahead to the next step (shaving). If you are replacing INE Transducer arrays,
you, or your doctor or caregiver if needed, should wipe the skin with baby oil to
remove any remaining adhesive from previous INE Transducer arrays. Baby oil is used
to remove remaining adhesive. It will not stop the device from working.
Shave your entire scalp using an electric shaver. Do not leave any stubble. Wipe your
scalp with 70% Alcohol (available at your local pharmacy without a prescription).
Use an over-the-counter hydrocortisone (steroid) cream if your scalp is red. Treat
open sores on your scalp like your doctor told you. If you use this cream, wait at least
15 minutes and wipe your scalp again with 70% Alcohol. Apply the INE Transducer
arrays after your scalp is dry.

18
11
Placing The INE Transducer Arrays On Your
Head
After you prepare your scalp (Section 9), put the INE Transducer arrays on your head
with the help of a doctor or caregiver if needed. Twice a week, remove the INE
Transducer arrays, prepare the scalp (as outlined in Section 13) and put on a new set
of INE Transducer arrays. You will know it is time to change INE Transducer arrays
when the device alarm beeps more often. This means that the device is not able to
work properly because of hair growth. Hair growth keeps the INE Transducer arrays
from making good contact with your scalp.
To place the INE Transducer arrays on your head, with the help of a caregiver or
doctor if needed, follow the steps below. Note, if this is the first time you have used
the INE Transducer arrays, ignore the first step (removal).
Remove the INE Transducer arrays from your head by peeling the medical tape away
from your scalp.
In the Treatment Kit, there are INE Transducer arrays having two colors of connectors
–black and white.
Note which color INE Transducer array goes where on your head. The INE Transducer
array locations and colors are: front & back (black), right & left (white).
Prepare your skin for the INE Transducer arrays, as described in Section 4.
Peel off the white layer (liner) covering the gel from the first INE Transducer array.
NOTICE: make sure there is no transparent cover with blue lines over the gel! In case
there is, carefully remove before proceeding.
If this is the first time you have used the INE Transducer arrays, put the INE
Transducer arrays on your head as shown in the INE Transducer array placement
diagram that your doctor gave you.
Placement is based on the location of your tumor. When changing the INE Transducer
arrays, place the INE Transducer arrays on your head in the same general location as
before, but shift the INE Transducer arrays about 2cm in the direction of the arrow on
your INE Transducer array placement diagram.
To reduce skin irritation under the INE Transducer arrays, move the INE Transducer
arrays a small amount. Place the other three INE Transducer arrays in the same way.
Pull the tabs on each side of the INE Transducer arrays and press them firmly to your
scalp. Press the entire edge of the INE Transducer array tape to your scalp.

19

20
12
Connecting the INE Transducer Arrays to the
Device
Connect each of the four INE Transducer array connectors with the black or white
connector to the matching color socket on the connection cable. For example, plug the
INE Transducer array with the black connector into the black socket (labeled “N1”; see
diagram).
Connect the other three INE Transducer array connectors in the same way.
Press firmly to be sure the connectors are pushed in all the way. Hold the INE
Transducer array wires together. Wrap them with a small piece of tape, if you wish.
You may clip the connection cable to your belt.
Refer to the Optune® Treatment Kit User Manual for instructions on how to start
treatment.
Other manuals for Optune NovoTTF 200A
1
Table of contents
Other novocure Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual