Melbea MelbeaGold Operating manual

T-shaped gold-copper alloy intrauterine
contraceptive medical device
INFORMATION
FOR THE USER:
Information for Women

2
INFORMATION FOR WOMEN
CONTENTS
0. PRELIMINARY INFORMATION
1. WHAT KIND OF PRODUCT IS MELBEAGOLD?
2. WHAT IS MELBEAGOLD COMPOSED OF? WHAT DOES IT
LOOK LIKE?
3. HOW DOES THE MELBEAGOLD PRODUCT ACHIEVE ITS
EFFECT?
3.1. What is the mechanism of action of the MelbeaGold
product?
3.2. How effective is the MelbeaGold product?
4. IN WHAT SIZES IS MELBEAGOLD AVAILABLE? FOR WHAT
UTERINE SIZES IS THE PRODUCT AVAILABLE?
5. HOW LONG CAN MELBEAGOLD BE USED AFTER
INSERTION?
5.1. What is the maximum lifetime in situ of the
MelbeaGold product?
5.2. What to do if you wish to continue the use of
MelbeaGold products for contraceptive purposes?
5.3. What to do if you wish to stop using your MelbeaGold
product before the end of its lifetime?
6. WHAT ARE THE INDICATIONS AND COUNTERINDICATIONS
OF USING MELBEAGOLD?
6.1. Who and in what cases should use MelbeaGold
products?
6.2. Absolute contraindications
6.3. Relative contraindications
7. WHAT WARNINGS SHOULD BE TAKEN INTO ACCOUNT
WHILE USING MELBEAGOLD?
8. WHAT POTENTIAL INTERACTIONS AND
INCOMPATIBILITIES ARE KNOWN BETWEEN MELBEAGOLD
AND OTHER MEDICAL TREATMENTS AND MEDICATIONS?
8.1. Interactions with medications and other devices
8.2. Potential interactions and incompatibilities with other
medical treatments

3
9. HOW TO INSERT AND REMOVE THE MELBEAGOLD
PRODUCT?
9.1. When is it recommended to insert the MelbeaGold
product?
9.2. Description of the insertion technique
9.3. Description of the removal technique
10. WHAT EFFECTS CAN THE USE OF MELBEAGOLD HAVE ON
THE MENSTRUATION CYCLE?
11. HOW TO VERIFY THAT THE MELBEAGOLD PRODUCT IS IN
AN APPROPRIATE POSITION DURING ITS USE?
12. WHEN IS IT RECOMMENDED TO SEE A GYNAECOLOGIST
FOR ROUTINE CHECK-UPS?
13. WHAT SIGNS AND SYMPTOMS INDICATE THAT YOU
SHOULD UNDERGO A GYNAECOLOGICAL EXAMINATION?
14. WHAT ARE THE POSSIBLE SIDE EFFECTS THAT MAY ARISE
DURING THE USE OF MELBEAGOLD?
15. MISCELLANEOUS INFORMATION
15.1. Manufacturer’s declarations
15.2. Details and contact information of the manufacturer
15.3. Version number and issue date of the document
15.4. Issue date of the first licence
15.5. CE identification number of the certification body
15.6. Description of the symbols used on the label
15.6.1.
Meaning of the symbols and pictograms used by
the manufacturer on the label
15.6.2.
Meaning of the symbols and pictograms used by
the manufacturer on the patient card

4
0. PRELIMINARY INFORMATION
Please read this information leaflet carefully before deciding if MelbeaGold
is right for you.
This information leaflet does not replace a consultation with your
gynaecologist or any other health care service provider specialising in
women’s health.
If you have any questions regarding the use of the MelbeaGold product,
please consult your doctor.
You need to be familiar with other contraceptive methods as well, since
the use of this product requires that you consult with your gynaecologist
and decide together which contraceptive method is the most optimal for
you, taking into account the results of a thorough medical examination.
1. WHAT KIND OF PRODUCT IS MELBEAGOLD?
The MelbeaGold product is what is
commonly known as
a hormone-free
copper coil, which is inserted by a gynaecologist into your uterine cavity to
provide long-term protection against unwanted pregnancies.
In technical language,
MelbeaGold is a copper-containing medical device,
which falls into the category of Intrauterine Contraceptive Devices
abbreviated as
IUD
or
IUCD).
MelbeaGold does not protect against HIV infections (AIDS) or any other
sexually transmitted infections (STIs)!
2. WHAT IS MELBEAGOLD COMPOSED OF? WHAT DOES IT LOOK
LIKE?
All MelbeaGold products are structured around a flexible small T-shaped
translucent milky white polyethylene frame.
Around this
underlying structure shaped like the cross-section of a lily flower
,
a high-purity orange-red
wire containing a copper-gold alloy
is wound up
spirally in a single layer.
To the lower end of the plastic frame, a
blue indicator string ending in two
separate threads
is attached.
This indicator string is composed of a single non-absorbent polyester-
based surgical thread.
The double-threaded end of the indictor string is the only part of the
MelbeaGold product that you might feel while MelbeaGold is in your uterus;
however, these dual threads do not extend outside the vagina and your body.
The frame of the MelbeaGold device does not contain a special X-ray contrast
agent (
e.g. barium sulphate
).
3. HOW DOES THE MELBEAGOLD PRODUCT ACHIEVE ITS EFFECT?
3.1. What is the mechanism of action of the MelbeaGold product?
After being inserted by your gynaecologist or health care service provider
,
the MelbeaGold product, which built around a polyethylene frame that is
soft to the touch and shaped like a lily flower, will fit into place in a natural
and unnoticeable manner and be effective from the first day following the
day of insertion.
MelbeaGold provides double protection: in addition to the known
foreign
body and copper ion effect
, it also provides
electrochemical
protection
against unwanted pregnancies.
Our current understanding is that copper-containing coils achieve their
effect by rendering sperms unsuitable for insemination and preventing the
implantation of any fertilised eggs.
5
MelbeaGold does not prevent the ovary from creating an egg every month
(
ovulation
).
In the MelbeaGold wire, the precious metal gold holds particles together
like mortar holds bricks together in the wall of a building. Therefore, wires
made of this alloy do not fracture during use but exert their effects intensively
and evenly over the course of their lifetime as opposed to traditional coils
containing only copper.
3.2. How effective is the MelbeaGold product?
Table 1 presents the chance of pregnancy in the case of women using various
methods of contraception with the help of the so-called
Pearl Index
(PI).
With the Pearl Index, we can assess how effective various forms of
contraception are and what the chance of avoiding pregnancy is (
The PI
shows what chance of pregnancy is if 100 women use the same method
over the course of 12 months
).
The table contains the contraceptive methods similar in terms of effectiveness.
The most effective methods can be found at the end/bottom of the table.
The square found at the beginning/top of the table shows the chance of
pregnancy in the case of women who do not use contraceptive methods/
devices and are actively trying to become pregnant.
The Pearl Index value of intercourse without protection is 85 (
that is out of
100 women, 85 becomes pregnant
).
In comparison, in the case of the typical use of copper-containing IUDs
(
such as the MelbeaGold product
), which fall in the category of methods,
the Pearl Index value is 0.8. This means that in the first year, out of 100
women, less than 1 become pregnant.
Table No. 1 – Effectiveness of various contraceptive methods and the
associated risk of pregnancy
Name of
method/device
Percentage of cases where
pregnancy occurs
Over the course of 1 year *
In the case of
typical use
In the case of
perfect
use
Women not using contraceptive methods 85.00 85.00
Interrupted intercourse 27.00 4.00
Natural methods of contraception 25.00 4.00
Diaphragm (plastic cap
placed on the cervix)
In women who
have given birth
32.00 26.00
In women who
have not given birth
16.00 9.00
Spermicides 19.00 18.00
Condom
female
21.00 5.00
male
15.00 2.00
Contraceptive patch 8.00 0.30
Pills (combined, oestrogen-free) 8.00 0.30
Vaginal ring (containing hormones) 8.00 0.30
Hormone injection 3.00 0.30
Copper-containing intrauterine devices (IUD) 0.80 0.20
Female sterilisation (tubal ligation) 0.50 0.50
Hormone-containing intrauterine devices (IUD) 0.20 0.20
Male sterilisation (vasectomy) 0.15 0.10
Hormone implant 0.05 0.05
* For easier comparison, we displayed whole numbers with two decimal
places as well.
6
After the implantation of a fertilised egg, the only option is the abortion of
the pregnancy, since no 100% effective method is known save for complete
abstinence from intercourse.
Since the Pearl Index value of copper-containing IUDs is between 0.2 and
0.8, the possibility of pregnancy must be taken into account even when
using such contraceptive devices.
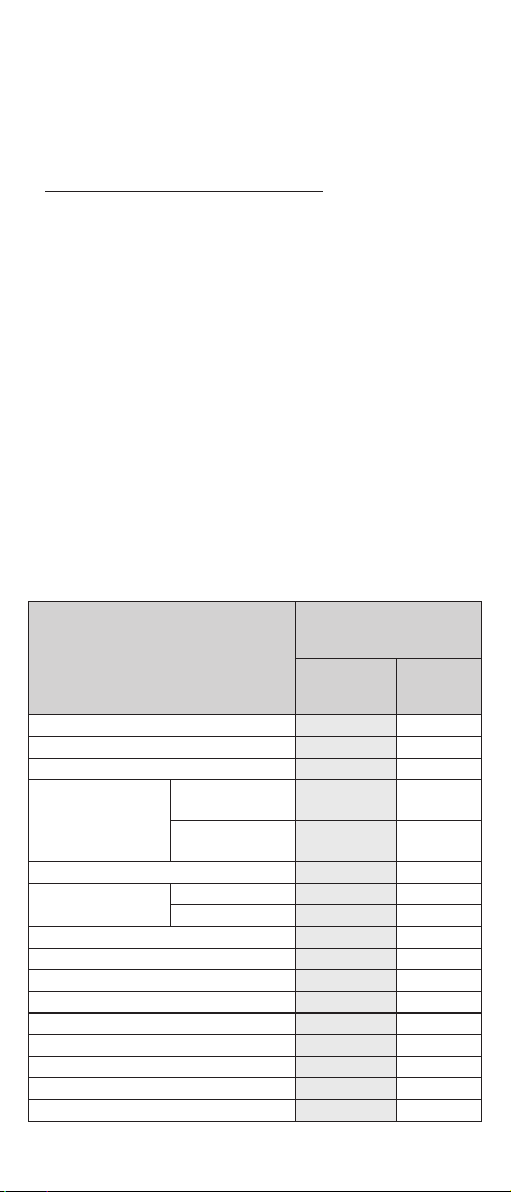
4. IN WHAT SIZES IS MELBEAGOLD AVAILABLE? FOR WHAT
UTERINE SIZES IS THE PRODUCT AVAILABLE?
Based on the size of the uterus, your gynaecologist or your health care service
provider can easily select the one most suitable for you from the mini, short,
and standard size variations of the MelbeaGold coil (see: Figure No. 1).
Figure No. 1 - the shapes, sizes, and ratios of the 3 size variations of the
MelbeaGold product
32 mm
STANDARD SHORT MINI
33 mm 26 mm
32 mm 24 mm
5. HOW LONG CAN MELBEAGOLD BE USED AFTER INSERTION?
5.1. What is the maximum lifetime in situ of the MelbeaGold product?
MelbeaGold protects against pregnancy for 5 years from the date of insertion.
5.2. What to do if you wish to continue the use of MelbeaGold products
for contraceptive purposes?
Your gynaecologist or health care service provider can insert another
MelbeaGold product during the same medical examination if you decide to
continue using MelbeaGold products for long-term contraception.
5.3. What to do if you wish to stop using your MelbeaGold product before
the end of its lifetime?
You can have your gynaecologist or health care service provider remove
the product at any time for any personal reason (
e.g. you decide that you
would like to become pregnant; you find certain side effects, such as heavier
bleeding, unacceptable; you would like to use a different method, your
MelbeaGold device was dislocated and removal or replacement is indicated;
the wrong size of the product was inserted and replacement is indicated
).
Fertility is restored immediately after the removal of the MelbeaGold device,
meaning you can become pregnant as soon as the product is removed. If
you change your mind after removal or you do not wish to become pregnant
in the future either, please use another contraceptive method, whichever is
most suitable for you, or another MelbeaGold product.
6. WHAT ARE THE INDICATIONS AND COUNTERINDICATIONS OF
USING MELBEAGOLD?
6.1. Who and in what cases should use MelbeaGold products?
We recommend the MelbeaGold product for you if
• you are looking for long-term birth control, which ensures that the chance
of becoming pregnant is low,
• you are looking for a reversible birth control method (
that retains its
contraceptive ability
),

7
• you are looking for a contraceptive method that you do not need to
re-apply every day,
• you are willing to use a contraceptive method that involves the insertion
of a device into the uterine cavity in spite of the risks,
• you are looking for a contraceptive method that does not contain
hormones,
• your current health conditions do not preclude the use of such a product
(see the contraindications under Sections 6.2 and 6.3).
There are health conditions in the case of which the use of copper-containing
coils is contraindicated. Either because it would not be safe or because there
is currently insufficient information about how safe it would be.
In the case of the presence or suspected presence of certain medical
conditions, the use of a copper-containing IUD, such as MelbeaGold, is
contraindicated (see
absolute contraindications
).
In the case of the presence of certain other conditions, your gynaecologist
or health care service provider may decide, having consulted you whether
MelbeaGold can be used and the insertion implemented, after which you
may agree to the insertion of the device at your own responsibility in spite
of the heightened risks (see
relative contraindications
).
6.2. Absolute contraindications
The MelbeaGold product must not be inserted in the following cases:
»Confirmed or suspected pregnancy,
»Your medical history includes ectopic pregnancy,
»Malignant disease or tumour in the reproductive organs, malignant
gestational trophoblastic disease (
cancerous diseases or lesions of
the reproductive system
),
»Congenital or acquired deformities of the uterus – any uterine condition
that affects the shape of the uterine cavity,
such as a large fibroid tumour,
retroverted uterus
,
»Abnormal (acyclic) uterine bleeding or bleeding of unknown origin in
the vagina,
»An infection of the uterus, the reproductive organs, or the pelvic organs
in the past 12 months (cervicitis, vaginitis,
especially bacterial vaginosis,
recurrent herpes infection or hepatitis B infection
),
»Pelvic tuberculosis or untreated acute pelvic inflammatory disease (PID),
»Inflammatory genital diseases,
including genital diseases of autoimmune
or unknown origin and multifactorial genital diseases
(
e.g. the long-
term use of a copper-containing IUD may be a risk factor in the case of
endometriosis, especially in the presence of adhesions
),
»Copper allergy, hypersensitivity to any component or material of the
product (
e.g. metal contaminants in the alloy such as nickel, or polyethene
plastic
), anaphylaxis (
a severe generalised potentially life-threatening
immunological hypersensitivity reaction of the body to a foreign material
)
or epileptic seizures (
a disorder of the nervous system that manifests
as a series of convulsive or nonconvulsive seizures
),
»Maternal sepsis (sometimes called
blood poisoning
) or abortion in the
last (
past
) three months.
»Sexually transmitted infections (
abbreviated as
STIs),
»An IUD or IUS inserted earlier which has not yet been removed from the
uterus of the patient.
Other conditions, in the presence of which the risks generally exceed the
advantages of using a copper-containing IUD, such as the MelbeaGold
device:

8
»Benign gestational trophoblastic disease (
proliferative disorders of the
reproductive system, non-cancerous diseases
),
»The period following childbirth (between 48 hours and 4 weeks),
»Ovarian cancer,
»High probability of exposure to infectious diseases such as AIDS,
gonorrhoea or chlamydia.
6.3. Relative contraindications
The use of the MelbeaGold product must be should be carefully considered
in the following cases:
»Heart valve diseases,
»Anemia, coagulation disorders (including the taking of anticoagulant
medication),
»Significant hyper- or dysmenorrhea (prolonged or painful menstruation),
»Cervical stenosis caused by scar tissue that renders insertion impossible,
»Bradycardia (cardiac arrhythmia associated with a low pulse), circulatory
disorders, vasovagal syncope (loss of consciousness due to hypotension
or heart failure),
»Undergoing treatment with an anti-inflammatory preparation,
»Wilson disease (a rare genetic disorder of the copper metabolism),
»Frequent change of sexual partners (either the patient or the patient’s
partner has multiple sexual partners),
»Conditions associated with increased susceptibility to pelvic infections,
»Women of childbearing potential who have not yet given birth (including
virgin women).
Taking the above into account, please inform your gynaecologist or
healthcare service provider if any one of the above health conditions
applies to you and if you have recently given birth or you are currently
breastfeeding.
7. WHAT WARNINGS SHOULD BE TAKEN INTO ACCOUNT WHILE
USING MELBEAGOLD?
–Your gynaecologist or health care service provider will give you a guide
titled “Information for women”.
Please carefully read and understand this guide.
–Please retain the “Information for women” guide and the “Patient Card”
filled out by your gynaecologist or health care service provider.
After you are given this card, please verify that all fields have been filled
out before leaving the medical facility.
–Medical devices are sometimes used for purposes not listed on the
information leaflet among the indications of the manufacturer or used
in spite of the existence of a relative contraindicated condition.
Please request further information from your gynaecologist or healthcare
service provider regarding the product details included in the user
instructions intended for medical professionals.
–MelbeaGold is a single-use device which comes in a sterile packaging;
the device must not be reused!
–The insertion and removal procedures may cause slight pain or bleeding.
The procedures may trigger fainting in the case of patients suffering
from circulatory disorders and seizures in the case of patients suffering
from epilepsy.

9
–Very rarely, directly after the insertion of copper-containing coils (such
as MelbeaGold), the patient may experience pain and dizziness.
If these symptoms persist after a 30-minute rest period, it is possible
that the position of the product inside the uterus is not suitable. In such
cases, the position of the IUD has to be checked and if it is not suitable,
the device must be removed.
It is recommended that you stay in the waiting room of your gynaecologist
or healthcare service provider for half an hour. You might be asked to
lay down until you feel better and to get up slowly afterwards. If you do
not experience these symptoms, feel free to leave the waiting room.
–Women may use the MelbeaGold product for contraception while
breastfeeding.
However, an increased risk of perforation was shown in the case of
breastfeeding women. If the device is inserted directly after childbirth,
the chance of the partial or complete perforation of the uterine wall is
higher due to the uterine wall being softer.
–MelbeaGold may trigger allergic reactions in hypersensitive individuals.
Before insertion, it is recommended to perform a test to confirm that you
are not allergic to copper, nickel, or plastics.
–You may use tampons while wearing the MelbeaGold device.
–The warnings related to the use of medications, other devices, and medical
treatments while wearing the MelbeaGold device are included in Section 8.
–Your doctor will inform you how you can check the presence of the
indicator string in your vagina; further details are included in Section 11.
–The warnings related to the possible side effects you may experience
while wearing the MelbeaGold device are included in Section 14.
8. WHAT POTENTIAL INTERACTIONS AND INCOMPATIBILITIES
ARE KNOWN BETWEEN MELBEAGOLD AND OTHER MEDICAL
TREATMENTS AND MEDICATIONS?
8.1. Interactions with medications and other devices
The MelbeaGold device and the equivalent Goldlily device have not undergone
an interaction study to identify possible interactions between the medical
devices and other treatments, medications, and devices.
Experience and the relevant scientific literature have shown that a reduction
in the effectiveness of copper-containing IUDs as a result of the use of
medication is unlikely.
However, notices contain information about a reduction in the effectiveness
of copper-containing IUDs as a result of the long-term use of non-steroidal
anti-inflammatory drugs (acetylsalicylic acid in particular) as well as
corticosteroids.
It appears that the
short-term use
of corticosteroids for the treatment of
period pain (dysmenorrhea) does not affect the contraceptive effectiveness
of copper-containing IUD.
8.2. Potential interactions and incompatibilities with other medical
treatments
–Short-wave diathermy of the pelvic region must not be performed while
the device is in place!
Medical devices generating high doses of radio frequency (RF) energy,
such as diathermy devices, may cause undesirable health effects in
the case of women wearing copper-containing IUDs, including the
MelbeaGold device, due to the heating of the tissues.

10
Make sure to inform your health care service provider that you have a
copper-containing intrauterine device (MelbeaGold) inserted if they are
planning to perform a short-wave treatment of the pelvic region, such as
diathermy, on you. Do not consent to such thermotherapy procedures
while the MelbeaGold device is within your uterus.
–The use of the MelbeaGold product in an MR environment may pose a risk!
The MelbeaGold device and the equivalent Goldlily device have not
undergone testing to establish whether a magnetic resonance (MR)
environment may cause such devices to heat up or become dislodged
and it has not been tested to what extent the assessment of the data
obtained during the imaging process may be affected by such devices.
However, based on data from scientific literature and non-clinical studies,
IUDs with copper-gold alloy wires are safe to use at specific MR values,
their shape and materials are not damaged or distorted, the devices do
not become dislodged, they do not heat up, or damage their environment.
Before undergoing an MRI procedure, please inform your health care
service provider that a copper-containing intrauterine device (MelbeaGold)
is within your uterus.
If the MRI system does not generate more than 3.0 T and the process
does not take more than 15 minutes, the removal of IUDs containing
copper or a copper-gold alloy is not required.
In the case of other MR imaging parameters, you must consult with the
physician conducting the examination and decide whether to remove
the device prior to the procedure, to use a different imaging method, or
to perform the imaging procedure with great care, under supervision,
and at your own risk.
9. HOW TO INSERT AND REMOVE THE MELBEAGOLD PRODUCT?
Before insertion, you must become familiar with the mechanism of action
of the MelbeaGold device, the risk posed by its use, the possible side
effects and their frequency, the possible time of their onset, and the relevant
symptoms and consequences.
You must undergo a gynaecological examination which includes abdominal
ultrasound and a cervical smear test as well.
The possibility of pregnancy, lower body infections, and sexually transmitted
diseases must be excluded.
It must be established whether you have any conditions which may fall
within the scope of contraindications.
Please inform your gynaecologist or health care service provider whether you
use any other contraceptive methods or devices (
e.g. whether you already
have an IUS in your uterus
) since this may qualify as a contraindicated
condition or the date of insertion may have to be changed in order to ensure
your continuous uninterrupted protection against unwanted pregnancies.
Your gynaecologist or health care service provider will first examine your
pelvic region to establish the exact position of the uterus.
9.1. When is it recommended to insert the MelbeaGold product?
–The device is primarily recommended to be inserted during the last days
of menstruation or within three days thereafter.
This ensures that the device can be worn successfully for a long period
and the probability of undiagnosed pregnancy can be reduced.
–The device may also be inserted directly after an abortion or miscarriage
or in the 10-15-minute period after giving birth.
However, the probability of rejection and perforation increases in such
cases.

11
MelbeaGold can be inserted immediately after an abortion performed
in the first trimester.
If the MelbeaGold device is not inserted within the first 15 minutes
after giving birth, the earliest it can be inserted is after the 4th week but
insertion is optimal and recommended from the 6th week onward since
the probability of perforation and rejection is high in the first 4 weeks.
–For the purpose of contraception, the IUD can be inserted within 5
days of unprotected intercourse on any day of the cycle (“emergency
contraception”).
In the case of such an insertion, the probability of acute pelvic inflammatory
disease (PID) may be higher than average.
9.2. Description of the insertion technique
Your gynaecologist or health care service provider will use an antiseptic
solution to clean your vagina and cervix then measures the length of your
uterus.
After this, the medical professional will slide a plastic tube containing the
MelbeaGold product into your uterus via the vagina and the cervix. Due to
the small diameter of the insertion tube, cervical dilation is not required.
When the MelbeaGold device enters your uterus, you might experience
cramps or a stinging sensation. Bleeding is also possible.
Your gynaecologist or health care service provider will remove the insertion
tube, leaving the MelbeaGold device in your uterus. Two threads of the blue
indicator string will extend into your vagina.
The threads will be cut so that they will be just long enough to allow you to
feel them with your fingers during a self-check.
If the device is pulled into the insertion tube and inserted in accordance
with the steps specified in the user instructions for medical professionals,
the correctly positioned T-shaped MelbeaGold device will be located in your
uterine cavity near the fundus.
The two arms of the product will be fully open on both sides, extending
toward the uterine horns.
The body of the IUD, together with the indicator string exiting via the cervix,
will extend into the lower portion of the uterine cavity to a smaller extent.
DEVICE (IUD)
Figure No. 2 – The position of the MelbeaGold product in the uterine cavity
(not to scale)

12
9.3. Description of the removal technique
–The most suitable time for the removal of a MelbeaGold product is
during menstruation.
–The MelbeaGold product is removed by gently pulling on the body of the
device while holding both threads of the indicator string.
The indicator string has to be held with a suitable tool (
e.g. sponge
forceps
) and carefully pulled out in the direction of the longitudinal axis
of the uterus.
As soon as the IUD appears in the cervix, grip the device with the sponge
forceps and continue pulling it until the entirety of the MelbeaGold product
has been removed.
Do not continue pulling the device by the indicator string until it is
completely removed since the string can snap and the end of the device
body may cause injury to the walls of the reproductive organs if it is
being pulled at an angle.
–If the strings are not visible but the MelbeaGold device is still in the
uterus, the removal must be postponed until the next menstruation.
When bleeding has ceased, the double threads of the indicator string
generally become visible again.
If the indicator string is still not visible and the MelbeaGold device is still
in the uterus, the device can be removed using a pair of thin forceps.
–Following the inspection of the removed MelbeaGold device by the
gynaecologist and if required, the taking of photographs or the recording
of video footage, the gynaecologist must ensure that the device, which
is considered infectious hazardous waste after removal, is placed in a
marked container, and disposed of as required.
10. WHAT EFFECTS CAN THE USE OF MELBEAGOLD HAVE ON THE
MENSTRUATION CYCLE?
The first menstrual cycles following insertion generally differ from previous
cycles.
The menses might be more abundant, heavier and might last longer; you
might also experience intermenstrual bleeding or abdominal cramps.
These are temporary phenomena; such symptoms are generally not a
cause for concern.
These symptoms may occur during menstruation or in the intermenstrual
period.
11. HOW TO VERIFY THAT THE MELBEAGOLD PRODUCT IS IN AN
APPROPRIATE POSITION DURING ITS USE?
You have to regularly verify whether the MelbeaGold device is still in your
uterus and whether it is in the correct position. You have to be taught the
correct method for finding the indicator string with your fingers by your
gynaecologist or healthcare service provider.
Clean and disinfect your hands before performing the self-check.
Assume a comfortable body posture (crouching, sitting, or similar).
With your clean and disinfected fingers, reach up to the upper part of the
vagina until you can feel the two threads.
Make sure that you can feel them but do not pull on them, since this could
cause the MelbeaGold device to become dislodged.
It is recommended that you check the position and length of the indicator
string at least once every month.

13
12. WHEN IS IT RECOMMENDED TO SEE A GYNAECOLOGIST FOR
ROUTINE CHECK-UPS?
Following the insertion of the MelbeaGold device, regular routine check-ups
are recommended even if the patient does not experience any symptoms
to ensure that the position of the product within the uterus can be verified
and the possibility of an infection, rejection, fracturing, or perforation can
be excluded.
You have to undergo a routine check-up following the first menstruation
after the insertion, that is in the 1st month of wearing the device, then in the
6th and the 12th months and at least once every year afterwards unless more
frequent examinations are required due to medical reasons.
If more frequent (
e.g. after every 6-8 weeks or in the 3
rd
month
) examinations
are required in your case, (
e.g. the MelbeaGold device is inserted in spite of
relative contraindications
), you will be informed of this by your gynaecologist
or healthcare service provider.
13. WHAT SIGNS AND SYMPTOMS INDICATE THAT YOU SHOULD
UNDERGO A GYNAECOLOGICAL EXAMINATION?
Always contact your gynaecologist or healthcare service provider if
• you believe that you are pregnant,
• you are experiencing pelvic pain or you experience pain during intercourse,
• you are noticing unusual vaginal discharge or genital sores,
• you are experiencing unexplained fever, flu-like symptoms, or body chills,
• you might be exposed to sexually transmitted infections,
• you are concerned that the MelbeaGold was rejected (
“came out
”), or
became broken (part of it was expelled),
• you cannot feel the indicator strings of the MelbeaGold device or you
feel them to be significantly longer than usual,
• you can feel any part of the MelbeaGold device in the vagina beside
the indicator string,
• you or your partner are found to be HIV positive,
• you are experiencing prolonged or heavy bleeding or any kind of bleeding
that worries you,
• you missed a menstrual cycle.
With respect to the background of the clinical symptoms and disorders
listed here, further clarification is provided in other chapters and sections
of the information leaflet.
–If you notice a change in the position or length of the indicator string
while performing a self-check:
Immediately report to your gynaecologist or healthcare service provider
if you notice a change in the length of the two threads, if you cannot find
the threads, or if you can feel any part of the MelbeaGold device in the
vagina other than the threads.
In such cases, the product might not be in a suitable position to prevent
unwanted pregnancies.
Use other contraceptive methods (
such as condoms or spermicide
preparations
) and ask your gynaecologist or healthcare service provider
to check whether the MelbeaGold device is in the correct position and
whether it is still in your uterine cavity.
–If the MelbeaGold product is accidentally removed:
If the product is accidentally removed, you are at risk of pregnancy; use
other contraceptive methods (
such as condoms or spermicide products
)

14
and immediately inform your gynaecologist or healthcare service provider
about the accidental removal of the MelbeaGold product.
Present the accidentally removed device to your doctor to allow them to
verify whether all components of the device are present and the device
is undamaged and to ensure that no parts of it broke off and remained
in the reproductive system.
The MelbeaGold device so removed must be considered potentially
infectious material; accordingly, you must ensure that it is taken to your
gynaecologist or healthcare service provider in appropriately sealed,
leakage-free packaging.
Following the inspection of the removed MelbeaGold device by the
gynaecologist and if required, the taking of photographs or the recording
of video footage, the gynaecologist must ensure that the device, which
is considered infectious hazardous waste after removal, is placed in a
marked container, and disposed of as required.
–If you are experiencing vaginal discharge or abdominal pain, immediately
visit your doctor.
–Contact your doctor immediately if you experience severe pain or fever
after the insertion of the MelbeaGold product.
–Visit your doctor if your menstrual cycle is delayed since it is rare but
possible to develop both intrauterine and ectopic pregnancy even in
spite of using the MelbeaGold device.
In the event of pregnancy, the MelbeaGold product must be removed by
the end of the third month if the indicator string is visible.
The removal of the MelbeaGold device during pregnancy may lead to
abortion.
Your gynaecologist or health care service provider will offer to abort
the pregnancy due to the increased risk of pelvic inflammatory disease
and other severe complications in such cases, including labour pains,
miscarriage, sepsis, and death.
If you decide to carry the pregnancy to term or the MelbeaGold device
can no longer be removed (
your pregnancy is in an advanced stage
),
you have to be informed in detail of the risks caused by the presence of
a MelbeaGold device which was not removed.
The risk of miscarriage is significantly higher in such cases than when the
coil is removed and you will require especially extensive prenatal care.
If you decide to carry the pregnancy to term, visit your doctor regularly.
Immediately contact or visit your gynaecologist or healthcare service
provider if you are experiencing any of the following symptoms:
–you experience flu-like symptoms, fever, body chills, cramps, pain,
bleeding, vaginal discharge, or fluid seeping from the vagina during
pregnancy. These could be signs of an infection.
–If the entirety of the MelbeaGold device is expelled from your uterus, the
device ceases to provide contraceptive protection. The risk of pregnancy
is present even in the case of a partial expulsion. Visit your doctor
immediately.
If the rejected device or a part of it is available, present it to your doctor
for inspection.
The MelbeaGold device so removed must be considered potentially
infectious material; accordingly, you must ensure that it is taken to your
gynaecologist or healthcare service provider in appropriately sealed,
leakage-free packaging.
Following the inspection of the removed MelbeaGold device by the
gynaecologist and if required, the taking of photographs or the recording

15
of video footage, the gynaecologist must ensure that the device, which
is considered infectious hazardous waste after removal, is placed in a
marked container, and disposed of as required.
–After the removal or spontaneous expulsion of the product, check the
MelbeaGold device to verify that neither of its arms remained in the
uterine cavity, the wire is not damaged or fractured, and the device as
a whole is undamaged.
If the MelbeaGold device was damaged (
became broken
) in the uterus,
its position has to be established using hysteroscopy, ultrasound, or
X-ray imaging; surgical intervention might be necessary.
–Call your gynaecologist or health care service provider if the heavier
bleeding typical after the insertion becomes more severe or prolonged
or the spotting bleeding continues.
–Visit your doctor if you or your partner experience pain during intercourse.
Neither you nor your partner is supposed to feel the MelbeaGold product
during intercourse.
The MelbeaGold product is inserted into the uterine cavity, not the vagina.
It is possible for your partner to feel the double ends of the indicator
string during intercourse.
–Please inform your gynaecologist or health care service provider of any
side effects that bother you as well as of any persistent side effects. Ask
your doctor about side effects.
You or your doctor may report side effects to the manufacturer directly
or using the dedicated medical device vigilance system.
In such cases, the device should be retained by your gynaecologist or
healthcare service provider in the manner required in the case of infectious
materials or at least take photographs or record video footage in order
to facilitate a successful investigation by the manufacturer and/or the
certification body or the competent authorities.
Experiencing a known side effect does not mean that the product is faulty.
–Immediately notify your gynaecologist or healthcare service provider if
you are experiencing any of the following symptoms:
–lower abdominal or pelvic pain, odourless discharge, unusual
vaginal discharge, unexplained or heavy or prolonged bleeding,
fever, body chills, the appearance of genital lesions or genital sores,
pain during intercourse, typically shortly after the insertion of the
MelbeaGold device.
These might also be the symptoms of pelvic inflammatory disease (PID),
endometritis, or resultant sepsis.
PID, endometritis, and sepsis require immediate treatment since untreated
PID or endometriosis may have severe consequences (
e.g. infertility,
damage to the fallopian tubes, hysterectomy
) and may cause death;
sepsis may also cause death.
–Immediately notify your gynaecologist or healthcare service provider if
you are experiencing any of the following symptoms:
Excessive pain or vaginal bleeding during the insertion of the MelbeaGold
device, pain or bleeding that becomes more severe after insertion, or if
you cannot find the indicator string.
These might be the symptoms of uterine perforation.
–Metal allergy (
e.g. caused by copper
) most commonly manifests in the
form of dermal symptoms.
Visit your gynaecologist or healthcare service provider if you are
experiencing any of the following symptoms:
16
–Redness, itching, swelling, flaky, dehydrated skin. Over a longer period,
the skin may become thicker and may develop cracks and keratosis.
Other symptoms may also occur, such as respiratory problems.
These might be caused by the copper released by the MelbeaGold
product. If you did not experience these symptoms before insertion,
you should definitely have yourself tested for copper allergy. If you are
allergic to copper, the product must be removed (
contraindication
).
The risk of developing a copper allergy is low since pure copper is
typically non-allergenic.
The alloy used in the MelbeaGold device consists of high purity
copper and gold.
–If you have any questions beside the above or you are uncertain about
any symptoms, please contact your gynaecologist or health care service
provider.
14. WHAT ARE THE POSSIBLE SIDE EFFECTS THAT MAY ARISE
DURING THE USE OF MELBEAGOLD?
The known side effects caused by the MelbeaGold product are the followings:
–pregnancy,
–rejection (complete or partial),
–uterine perforation (complete or partial perforation or implantation),
–breakage of the device,
–anaemia (low red blood cell count),
–pain during intercourse,
–spotting or heavy bleeding, bloodstains,
–prolonged menstrual periods,
–painful menstrual periods, including cramps,
–vaginal irritation or discharge,
–allergy.
This list of the possible side effects of the MelbeaGold product is non-
exhaustive. Please contact your gynaecologist or health care service
provider for further information.
Overview of the possible serious side effects that may be caused by the
MelbeaGold device or its use:
–Ectopic or intrauterine pregnancy
If you become pregnant during the use of the MelbeaGold device, the
chance of ectopic pregnancy is exceedingly high. This means that the
pregnancy occurs outside the womb.
Unusual vaginal bleeding or abdominal pain, especially in combination
with a missed menstrual cycle, may be symptoms of an ectopic pregnancy.
An ectopic pregnancy may cause internal bleeding, infertility, and
even death.
Intrauterine pregnancy also presents risks. As the uterus grows during
gestation, the indicator string of the MelbeaGold device is pulled into
the uterine cavity, thus allowing pathogenic microorganisms to enter
the uterus, possibly causing infection, spontaneous abortion, sepsis,
and even death.
Becoming pregnant while using copper-containing coils is a medical
emergency which often warrants surgery.
Based on our current understanding, the copper-containing coils
themselves, including MelbeaGold, do not cause harm to the foetus.
–Life-threatening infection

17
It is possible to develop a life-threatening infection in the first few days
following the insertion of the MelbeaGold device, the symptoms of which
may be fever, body chills, and pain.
–Acute pelvic inflammatory disease (PID), endometritis, and actinomycosis
In the case of some IUD users, a serious pelvic inflammatory disease
(PID) or endometritis may develop, which are usually sexually transmitted.
PID is an infection of the uterus, the fallopian tubes, and the adjacent
organs.
Among the types of human actinomycosis, pelvic actinomycosis is a
very rare disease, which is known to be associated with intrauterine,
including copper-containing, contraceptive devices and involves an
infection that spreads downward from the uterus.
In the case of MelbeaGold, it is most likely to occur in the first week
following insertion, accompanied by fever, pain, and bleeding. It is
hypothesised that the disease is caused by microorganisms entering
the uterine cavity during the insertion process and causing an infection.
During insertion, your gynaecologist or health care service provider
must strictly follow the steps of the aseptic insertion technique in order
to prevent and avoid infection.
Women who have suffered from PID or endometritis are exposed to a
higher risk of reinfection.
The probability of developing PID or endometritis is also higher if you or
your partner have multiple sexual partners.
PID, endometritis, and actinomycosis are treated with antibiotics; in
the case of more serious cases, surgery, or the removal of the womb
(hysterectomy) might be necessary.
In the case of such infections, the MelbeaGold product must be removed.
PID might cause serious problems such as infertility, an ectopic pregnancy,
and chronic pelvic pain. PID may also develop without symptoms! In rare
cases, PID might cause death.
–Rejection
The MelbeaGold device may spontaneously become dislodged and
fully or partially exit the uterus by itself. You are not protected against
pregnancy if the MelbeaGold device was rejected. Rejection occurs in
1-2 cases out of 100 women.
A significant precursor to rejection is the device becoming dislodged due to
the strong cramps or low-intensity but frequent contractions of the uterus.
In addition to rejection, these uterine contractions may also cause the
spontaneous breaking of the device or lead to the device becoming
dislodged and being ejected through the breaking of the product.
If you experience excessive pain or vaginal bleeding immediately during
the insertion of the MelbeaGold device, pain or bleeding that becomes
more severe after insertion, or if you cannot find the indicator string, the
device might have been rejected.
–Uterine perforation
T-shaped intrauterine contraceptive devices, such as the MelbeaGold
product, might puncture the uterine wall.
The perforation may be partial (
the majority of the coil remains in the
uterus
) or complete (
the coil is removed from the uterus and enters the
abdominal cavity
).
If this occurs, the MelbeaGold device is no longer able to prevent
pregnancy.

18
In the case of perforation, the MelbeaGold device may cause internal
scarring, infection, damage to other organs, pain, infertility, and even
death. In addition to pharmacotherapy, surgery is required to remove
the MelbeaGold device and treat any damaged organs.
In the case of breastfeeding women, women who have not given birth,
and women who have recently given birth, the risk of perforation is higher
when inserting the MelbeaGold device. If the device size is selected
incorrectly, this may also cause perforation.
The probability of perforation is higher in the case of women with
abnormal (
retroverted
) uteri or immobile uteri due to adhesions (
e.g.
caused by endometriosis
).
The uterine wall may be perforated by the physician during the insertion
or removal of the device (iatrogenic perforation) or at any time during or
after insertion due to forces caused by sudden and strong or weaker but
repeated uterine contractions (spontaneous perforation).
Id diagnosed early, the complications of perforation may be treated with
medication or surgery; fertility generally remains unaffected in such
cases. The MelbeaGold product must be removed.
–Implantation
In rare cases, the implantation of the device into the uterine wall may
make removal more difficult. In such cases, surgery might be required.
–Breaking of the product
In rare cases, the breaking of the MelbeaGold and pieces remaining
in the uterine cavity may make removal more difficult. In such cases,
surgery might be required. It is also possible that surgery will not be
required since all pieces of the product are rejected either at the time of
breaking or sometime afterwards.
–Changes to the menstrual cycle
You might experience heavier or longer menstrual cycles or spotting during
your period. Bleeding occasionally becomes heavier after insertion. In
abnormal cases, this bleeding may become even heavier and prolonged;
the number of bloodstains may increase.
–Unique arousal symptoms during the insertion or removal of the device
or thereafter:
In the case of some women, symptoms such as dizziness, low heart
rate, or cramps may present themselves after the insertion or removal
of the MelbeaGold device.
These symptoms may primarily be experienced by women who have
suffered from such symptoms before for any reason.

19
15. MISCELLANEOUS INFORMATION
15.1. Manufacturer’s Declarations
The manufacturer declares that MelbeaGold (
device and accessories
) do
not contain the following:
–materials, tissues, products of animal origin,
–human blood derivatives or products containing human blood derivatives,
–carcinogenic, mutagenic, teratogenic materials, including phthalates,
and products containing such materials,
–radioactive materials, GMOs, or products containing such materials,
–natural latex rubber or materials containing natural latex rubber.
The manufacturer declares that the packaging of MelbeaGold does not
contain the following: radioactive material.
15.2. Data and contact details of the manufacturer
MELBEA Innovations Kft.
H-6600 Szentes, Bese László u. 8. (Hungary)
www.melbea.com
15.3. Version number and issue date of the document
vMG-26.11.2020-EN, v03
15.4. Issue date of the first licence
18.07.2014
15.5. CE identification number of the certification body
2409
15.6. Description of the symbols used on the label
15.6.1
Meaning of the symbols and pictograms used by the
manufacturer on the label
Product sterilised with ethylene oxide
Manufacturing date (year, month)
Date of expiry (year, month)
Lot No. – unique product identifier
STERILIZE
Do not resterilise
Keep dry
2409 CE marking
Do not reuse
Upper limit of storage temperature
Always follow the instructions in the manual
DO NOT BEND OR SQUEEZE!
KEEP AWAY FROM CHILDREN!
Do not use product if damaged.
Fragile. handle with care.
Manufacturer
Contact details of the manufacturer
MR MR conditional
The label contains the identification number of the label (see:
LS-xxx
), its
version number (see:
vMG-xx.yy.zzzz
), a code indicating the size variation
of the device, as well as the country code of the language used (
e.g.: HU-ST
refers to the Hungarian version and the standard size variation of the product
).
15.6.2
Meaning of the symbols and pictograms used by the
manufacturer on the patient card
MANUFACTURER
CONTACT DETAILS OF THE MANUFACTURER
?PATIENT NAME
MD PRODUCT NAME
LOT
31 TIME AND DATE OF INSERTION
NAME OF INSERTING PHYSICIAN/INSTITUTION
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











