METRON ACCUSONIC PLUS User manual

Metron Medical Australia Pty Ltd
A.C.N.050 240 527
P.O. Box 2164,
57 Aster Avenue
Carrum Downs Victoria Australia 3201
Tel: (03) 9775 1234 Fax: (03) 9775 1990
Int: 61 3 9775 1234 Int: 61 3 9775 1990
TECHNICAL MANUAL
METRON ACCUSONIC PLUS
ULTRASOUND THERAPY UNIT
MODEL AP 100
Prepared by
Metron Medical Australia P/L
Version 1.6 March 2002

Metron Accusonic Plus
Metron Medical Australia Pty Ltd
A.C.N.050 240 527
P.O. Box 2164,
57 Aster Avenue
Carrum Downs Victoria Australia 3201
Tel: (03) 9775 1234 Fax: (03) 9775 1990
Int: 61 3 9775 1234 Int: 61 3 9775 1990
EC DECLARATION OF CONFORMITY
Metron Medical Australia Pty Ltd
57 Aster Avenue
Carrum Downs, Australia, 3201
declares that the medical devices described hereafter:
Metron Accusonic Plus Ultrasound Therapy Unit
Model: AP 100
is in conformity with the essential requirements and provisions of Council Directive
93/42/EEC
is subject to the procedure set out in Annex II of Directive 93/42/EEC under the
supervision of Notified Body Number 0120, SGS Yarsley International Certification
Services Ltd, Portland Road, East Grimstead, W Sussex RH19 4ET.
Melbourne, 23 August 1999
R. H. Hopkins Technical Director
On Behalf Of
Metron Medical Australia Pty Ltd
__________________________________________________________________
Version: 1.6 March 2002
Metron Accusonic Plus
0120
The Metron Accusonic Plus Ultrasound Therapy Unit bears the above marking in
accordance with the requirements of Council Directive 93/42/EEC.
Should you as the user of this technical manual wish to make any comment about the
product or this manual our Authorised Representative within the European Union may
be contacted as follows:
Metron Medical
Attention: Mrs Sue Marks
P.O. Box 5399
Southend-On-Sea
ESSEX SS1 3RY UNITED KINGDOM
__________________________________________________________
Version: 1.6 March 2002

Metron________________________________________Accusonic Plus
CONTENTS
Section Page
1. Specifications .............................................................................................. 1
2. Introduction .................................................................................................. 4
3. Schematic Diagram Descriptions ............................................................ 6
3.1 Printed Circuit Board ...................................................................... 6
3.1.1 Main Power Supply............................................................. 6
3.1.2 Oscillator, Output Stage & Contact Sense ..................... 6
3.1.3 Microcontroller...................................................................... 6
3.1.4 Displays & Drivers ............................................................... 7
3.2 Ultrasonic Treatment Applicator/s ................................................ 7
4. Preventative Maintenance / Quality Assurance....................................... 8
4.1 Calibration and Adjustment ............................................................ 8
4.1.1 Equipment Required ............................................................ 8
4.1.2 Calibration Procedure ......................................................... 8
4.2 Electrical Safety Inspection ............................................................. 9
4.3 Disassembling/Assembling the Unit ............................................. 9
5. Schematic Diagrams ................................................................................... 10
5.1 Main Circuit Board Layout .............................................................. 10
5.2Main Circuit Board Schematic Diagram ...................................... 11
5.3 Treatment Head Circuit Board Schematic Diagram .................. 12
__________________________________________________________________
Version 1.6 March 2002

1. SPECIFICATIONS
MAINS SUPPLY REQUIREMENTS:
Voltage 110 - 120 Volts AC or
220 - 240 Volts AC
Frequency 50/60 Hz
Power 75VA Maximum
FUSES:
Primary external 2 of 1A 5 X 20 mm DA205
Internal Fuse 1 of 4A 5x20mm DA205
POWER SUPPLY:
Integrated switchmode power supply complying with IEC 601-1: 1988 and
amendments.
Secondary Voltages 24 Volts DC @ 2.7A
ULTRASOUND OUTPUT - 1 MHz:
Frequency 1.1 MHz +/- 10%
Output Intensity, Continuous Mode 3.0 Watts/cm2+/- 20% Maximum
Power/Intensity Display Accurate to +/- 20% of reading for
outputs in excess of 0.2 Watts/cm2.
Effective Radiating Area 5.0 square cms +/- 20%.
ULTRASOUND OUTPUT - 3 MHz:
Frequency 3.3 MHz +/- 10%
Output power in continuous mode 3.0 Watts/cm2+/- 10% maximum
(Equivalent to 2.4 watts absolute for the
1cm head)
1.6 Watts/cm2+/- 10% maximum
(Equivalent to 8.0 watts absolute for the
2.5 cm head)
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 1 of 12
Effective Radiating Area Nominally 0.8 square cms +/- 20%
(1 cm head)
Nominally 5.0 square cms +/- 20%
(2.5 cm head)
Power/Intensity Display Accurate to +/- 10% of reading for
outputs in excess of 1 Watt.
ULTRASOUND MODULATION:
Modulation Modes Continuous
Pulsed
Pulsed Modulation
Pulse Frequency 100 Hz +/- 2%
Pulse Width 1.0, 2.0, 5.0 milliseconds +/- 2%
Pulse Duty Cycle 1:9 (10%), 1:4 (20%), 1:1 (50%)
respectively
BEAM NON-UNIFORMITY RATIO: 5:1 +/- 20%
TREATMENT TIMER:
Maximum Treatment Time 30 minutes +/- 2%
At Time Expiration Time display zeros and a 3 second
audible alarm sounds.
CONTACT CONTROL:
Function To detect poor acoustic coupling between the
ultrasonic treatment applicator and the patient.
On detection of contact loss The LED indicator on the selected applicator
handle turns red and, after a 2 second delay, the
treatment timer is halted. The ultrasonic output is
reduced to 0.2 Watts/cm2.
On detection of contact The treatment timer is restarted and the ultrasonic
output power is restored to the selected value.
The LED indicator on the applicator handle turns
green.
Purpose/Rationale Ensures the patient receives the required
ultrasound dose and prevents damage to the
ultrasonic treatment applicator by heat generated
in the transducer when it is operated unloaded at
high power levels.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 2 of 12

ELECTRICAL SAFETY:
Designed and manufactured to comply with the following Australian and International
standards:
AS 3100 - 1985 Definitions and general requirements for electrical materials
and equipment.
AS 3200.1 - 1990 Approval and test specification - Medical electrical equipment,
Part 1 General requirements for safety.
AS 3200.2.5 - 1992 Approval and test specification - Medical electrical equipment,
Part 2: Particular requirements for safety - Ultrasonic therapy
equipment.
IEC 601-1 - 1988 Medical electrical equipment, Part 1: General requirements.
IEC 601-2-5 - 1984 Medical electrical equipment, Part 2: Particular requirements for
the safety of ultrasonic therapy equipment.
Applied part Treatment applicator/s
Applied part classification BF
Chassis classification 1
DIMENSIONS:
Width 170 mm
Height 120 mm
Depth 280 mm
WEIGHT:
Packed 3 Kg
Unpacked 2.5 Kg
ENVIRONMENTAL CONDITIONS:
Operating: Temperature Range 10 - 40 oC
Relative Humidity 30% - 90%
Transport & Storage: Temperature Range 0 - 70 oC
Relative Humidity 10% - 100%
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 3 of 12

2. INTRODUCTION
This manual presents all the relevant technical information for the Metron Accusonic
Plus Ultrasound Therapy Unit. This information is provided as a service to medical,
paramedical, engineering and technical personnel. This information is intended for the
fair purposes of evaluation, maintenance and repair of the Accusonic. It is provided as
commercial-in-confidence material to the distributor or equipment purchaser and shall
not be made available to any other organisation or person without the specific written
permission of Metron Medical Australia Pty Ltd. Refer to the Metron Accusonic Plus
Operator Manual for operator information.
All functions of the schematic diagram are described. Necessary preventative
maintenance calibration adjustments are described in detail. Recommended electrical
safety inspection procedures are discussed.
While every attempt has been made to ensure that this manual is accurate and
complete, no responsibility is taken for any errors or omissions. Specifications and
component types are subject to change without notice.
If you, as a user of this manual, have any relevant comments or questions, your
communication with us would be welcomed. You may contact us by mail or fax as
detailed below:
Metron Medical Australia Pty Ltd
P.O. Box 2164
CARRUM DOWNS AUSTRALIA 3201
Fax (03) 9775 1990 from within Australia
+61 3 9775 1990 International.
The Accusonic Plus generates continuous and pulsed wave ultrasound. The ultrasonic
transducer in the ultrasonic treatment applicator is driven by an approximately
sinusoidal voltage derived from a micro-computer generated 1 MHz or 3 MHz signal
and tuned power amplifier. The amplitude of the transducer drive voltage determines
the ultrasonic power output. This power output is controlled by the power supply
voltage to the tuned power amplifier. The ultrasonic power output is proportional to the
square of the power supply voltage. The necessary parameters to control the supply
voltage to appropriate levels are stored in the connector of the treatment applicators.
The power supply voltage is generated by a programmable power supply which
employs a monolithic switch - mode voltage regulator. This supply is controlled by a
pulse width modulated signal generated by the microcontroller.
This microcontroller also performs a number of other operations crucial to the device. It
processes all the keyboard functions and drives all the 7 segment displays. It also acts
as the treatment timer and drives the audible alarm.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 4 of 12

The degree of acoustic coupling between the ultrasonic treatment applicator and the
patient is monitored by the contact sense circuit and the microcontroller. At detection of
contact loss the indicator on the selected applicator turns RED, the treatment timer
is halted and after 2 seconds of contact loss the ultrasonic output power is reduced from
that selected to 0.2 Watts/cm2. At detection of contact the treatment timer resumes and
the ultrasonic output power is restored to the selected value.
This ensures the patient receives the ultrasound dose selected and prevents damage
to the ultrasonic treatment applicator by the heat that would be generated in the
ultrasonic transducer if it was operated unloaded at high power levels.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 5 of 12

3. SCHEMATIC DIAGRAM DESCRIPTION
3.1 PRINTED CIRCUIT BOARD (see Schematic, page 11)
3.1.1 Main Power Supply.
The DC voltage from the switchmode power supply is applied to the electronics
through the diode bridge BR1. The bridge provides a level of protection against
wiring errors. The 24 volt DC supply supplies power to switching regulators
IC11, & IC12 and the Piezo speaker.
Switching regulator IC11 supplies +5 volt rail at 1A. Switching regulator IC12 is
the variable voltage supply. This supply is used to power the output amplifier
from 0 to +25 volts DC at 3A. The power supplies output voltage is controlled by
the microcontroller via filtering components R2, C29 & R5.
3.1.2 Oscillator, Output Stage & Contact Sense Control.
IC7 & IC8 form a PLL up convertor to generate the 1MHz signal for the output
stage. An input frequency of 61Hz is applied to pin 14 of IC7 from the
microcontroller and is multiplied by the PLL circuit 16385 times to a value of
1MHz or 3 MHz on pin 4 of IC9. IC10B is used to gate this signal for pulsed
mode. This signal is than buffered by IC9 to drive Q1 the output stage.
Alignment of the treatment applicators is controlled by the microcontroller and the
tune point of the applicators is stored in the eeprom located in the connector of
the treatment applicator.
L5 monitors supply current level in the output stage. Comparing this value with
preset values stored in eeprom, signals an in-contact or contact loss. If an
contact loss condition is encountered, the microcontroller reduces the output
power to 1W total (0.2 Watts/cm2) and pauses the timer. When contact is
reestablished the timer is restarted & power level is restored to that selected.
IC10A, IC10B & IC10C drive the bi-colour LEDs in the treatment applicators for
contact status.
Two output connectors are provided, J1 and J2, and the selection of one
particular connector is controlled by the “Applicator” key on the front panel and
the microprocessor. Relays RL1 and RL2 provide the necessary switching.
3.1.3 Microcontroller
IC13 resets the microcontroller IC1 on power up. The control program is stored
in memory IC3 and IC2 provides the address latching for IC3. IC4 provides the
external memory enable control.
The microcontroller monitors the keys and contact status and controls the drive
signal frequency, the seven segment displays, the piezo speaker drive and the
treatment applicators power output.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 6 of 12

3.1.4 Displays & Drivers
The seven segment displays, LED 11 and LED 12, and the individual LEDs,
LED1 through LED8, are driven by display drivers chips IC5 and IC6. These 35
segment drivers and controlled by serial information sent from the
microprocessor to their CK and DATA pins. The display intensity is determined
by R11 and R17. capacitors C25 and C28 provide filtering and voltage
stabilisation.
3.2 ULTRASONIC TREATMENT APPLICATOR/S
(see Schematic page 12)
The ultrasonic treatment applicator consists of a plastic and aluminium assembly
with the ultrasonic transducer bonded in position. The contact loss
indicator LED is driven by the LED signal from the contact sense circuit on the
main PCB. The LED has a capacitor connected in parallel with it to prevent the
transducer drive RF from turning it on.
The piezoelectric resonator which generates the ultrasound energy is
permanently bonded to the cap of the applicator. It can be replaced by simply
unscrewing the cup and resonator and attaching a new cup and resonator.
The connector of the treatment applicator contains a small printed circuit board
which houses IC1 and filter capacitor C1. IC1 is an EEPROM which is
programmed during the calibration sequence, see page 8, and stores the critical
information relating to optimum tune frequency and supply voltage levels for
each output setting necessary to maintain calibration.
This symbol indicates that the applied parts (treatment
applicators) of this equipment are rated as Type “BF”. This
means that the patient applied parts are suitable for
placement on the external surface of the patient without
creating a safety hazard.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 7 of 12

4. PREVENTATIVE MAINTENANCE / QUALITY ASSURANCE
4.1 Calibration and Adjustment
4.1.1 Equipment Required
The following equipment is a minimum requirement for the calibration of the
Metron Accusonic Plus.
Ultrasound Power Meter: Ohmic Instruments UPM-30
Bio-Tek Instruments UW-II
UMA Inc UMT-2A or equivalent
4.1.2 Calibration Procedure
Connect the applicator to be calibrated to either input on the Accusonic Plus.
NOTE: It is recommended that treatment applicators only be attached and
removed from the unit when the power is turned OFF.
Calibration mode can only be accessed by pressing the “Timer Down” button
and “Mode” button at the same time and then turning the power ON. This will
place the unit into alignment mode (Timer will display an "F"). For the 3 MHz 5
sqcm head select “LF” using the “Timer Down” button.
The first step is to align the frequency of the selected applicator. Remove any
water, gel etc from face of the applicator and place in free air (face of applicator
not in contact with anything). Select the frequency, using the “Timer Up” button,
press the “Mode” button and wait until the power display counts from 0 to 99.
When completed the timer display will show a "P" indicating the next step, the
alignment of the applicator’s output power and contact sense control, is ready to
be undertaken. Place the applicator in the power meter.
Press the “Mode” button and adjust the applicator output power, as displayed,
using the “Power Up/Down” buttons to match the power output displayed on the
ultrasound power meter. The first calibration point takes place with a mid point
output power setting, 8 watts (1 MHz 5sqcm), 5 watts (3 MHz 5 sqcm) and 1.3
watts (3 MHz 0.75 sqcm) . When this adjustment is completed press the “Mode”
button to move on to the next power level for adjustment.
Repeat the above procedure for the other two power settings, 2 and 15 watts
(1MHz 5 sqcm), 0.3 and 2.4 watts (3MHz 0.75 sqcm) or 2 and 10 watts (3MHz 5
sqcm) then press the “Mode” button again. This will initiate the contact sense
alignment mode which automatically scans each power level for each pulse
mode to determine the contact parameters. The treatment applicator should not
be removed from the ultrasound power meter until this procedure has finished.
The calibration procedure for that particular treatment applicator is now
completed. If another applicator needs calibration repeat the above procedure.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 8 of 12

At the completion of the calibration procedure it is required that the mains power
be turned OFF and then turned ON again to return the Accusonic Plus to the
normal operation mode.
WARNING: If the mains power is removed or turned OFF prior to the
completion of the calibration of an applicator the whole procedure
will need to be repeated.
4.2 Electrical Safety Inspection
A program of regular electrical safety inspections for this equipment is
recommended. The type and frequency of testing may be obtained from locally
published standards. In Australia, the relevant standards are:
AS 3511 - 1988 Acceptance testing and in-service testing -
Electromedical equipment
AS 2500 - 1986 Guide to the safe use of electricity in patient care.
A hospital biomedical engineering department or third party service organisation
nominated by the manufacturer or distributor would be capable of performing the
necessary testing and providing suitable documentation.
Programmed electrical safety inspections are recommended to confirm
continued operator and patient safety. Other mandatory statutory requirements
for electrical safety inspections may also apply.
4.3 Disassembling/Assembling the Unit
If required disassembly and assembly of the unit should be undertaken with
extreme care to avoid damage to the surfaces of the enclosure. Whenever the
unit has to be turned upside down, place it on a soft surface or thick cloth.
Disassembly of the unit is as follows:
-Place the unit upside down on a soft cloth. Remove the four pozi-
head screws around the perimeter of the base. Two are located
close to the front edge of the base and two are located close to the
rear edge of the base.
-Turn the unit up the right way and carefully lift the top away from the
base. The two halves can now be separated sufficiently to allow
access to all components.
Assembly is a reverse procedure of the above with several precautions. They
are: -Observe that any connectors, cables or wires that were removed
are correctly reinstated and are not fouled or crushed.
-Avoid over tightening the screws which secure the top to the base.
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 9 of 12
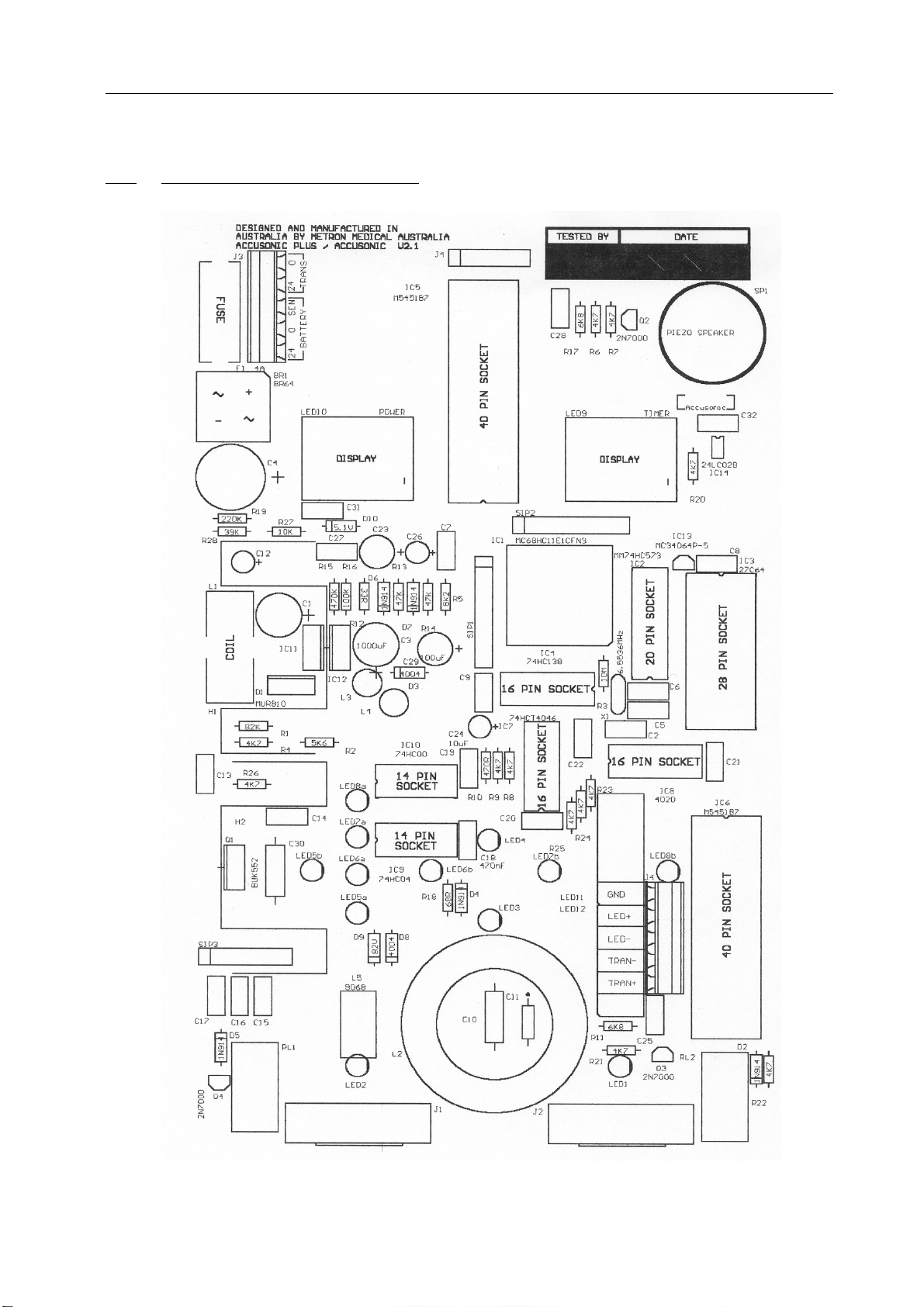
5. SCHEMATIC DIAGRAMS
5.1 Main Circuit Board Layout
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 10 of 12

5.2 Schematic Diagram - Main Printed Circuit Board
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 11 of 12

5.3 Schematic Diagram - Treatment Head Printed Circuit Board
Metron Accusonic Plus
__________________________________________________________________
Version 1.6 March 2002 Page 12 of 12
This manual suits for next models
1
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











