Millipore Sigma SNAP i.d. 2.0 User manual

The life science business of
Merck KGaA, Darmstadt, Germany
operates as MilliporeSigma in
the US and Canada.
www.sigmaaldrich.com
User Guide
SNAP i.d.® 2.0
Protein Detection System
for Western Blotting

CONTENTS
Introduction .........................................................................................................................1
SNAP i.d.®2.0 Protein Detection System Features ....................................................................................1
Parts and Functions of the SNAP i.d.®2.0 System .................................................................2
Symbols Used in this User Guide...........................................................................................3
Materials Required but Not Supplied.....................................................................................3
General Guidelines ...............................................................................................................4
Optimization Guidelines........................................................................................................5
Antibodies ..........................................................................................................................................5
Blot Blocking ........................................................................................................................7
How to Use the SNAP i.d.®2.0 System ..................................................................................8
System Setup......................................................................................................................................8
Blot Assembly and General Protocol........................................................................................................9
Extended Primary Antibody Incubation: One Hour to Overnight .........................................11
Antibody Recovery..............................................................................................................11
Proof of Performance..........................................................................................................12
Maintaining the SNAP i.d.®2.0 System................................................................................14
Cleaning Protocol ...............................................................................................................................14
Component Re-use.............................................................................................................................14
Troubleshooting .................................................................................................................15
Specifications .....................................................................................................................16
Chemical Compatibility .......................................................................................................16
Storage ..............................................................................................................................16
Ordering Information .........................................................................................................17
Notice.................................................................................................................................19
Contact Information ...........................................................................................................19
Technical Assistance...........................................................................................................19
Standard Warranty .............................................................................................................19
Conformance to Pressure Equipment Directive ...................................................................19
00000825W REV 05/19 1 of 19
Introduction
The new and improved SNAP i.d.®2.0 Protein Detection System is the second generation of the SNAP i.d.®method
for detecting immunoreactive proteins on Western blots. With this unique vacuum-driven system, the length of
time required for immunodetection is greatly reduced. What previously took 4 to 24 hours with traditional Western
blotting methods now takes only 30 minutes with no loss of signal intensity or reduction in blot quality. All
immunodetection steps after protein transfer to a membrane (i.e., blocking, washing, and primary and secondary
antibody incubations) can be performed with the SNAP i.d.®2.0 system.
Sample Prep
0.5–2
hours
0.5–1
hours
1–2.5
hours
1 hour 3 hours 15 minutes
Electro-
phoresis
Membrane
Transfer Blocking
Antibody
Incubation Detection
SNAP i.d.®2.0 System
30 minutes
SNAP i.d.®2.0 Protein Detection System Features
• Processing of up to four blots at a time on a vacuum base with two individually controlled sides
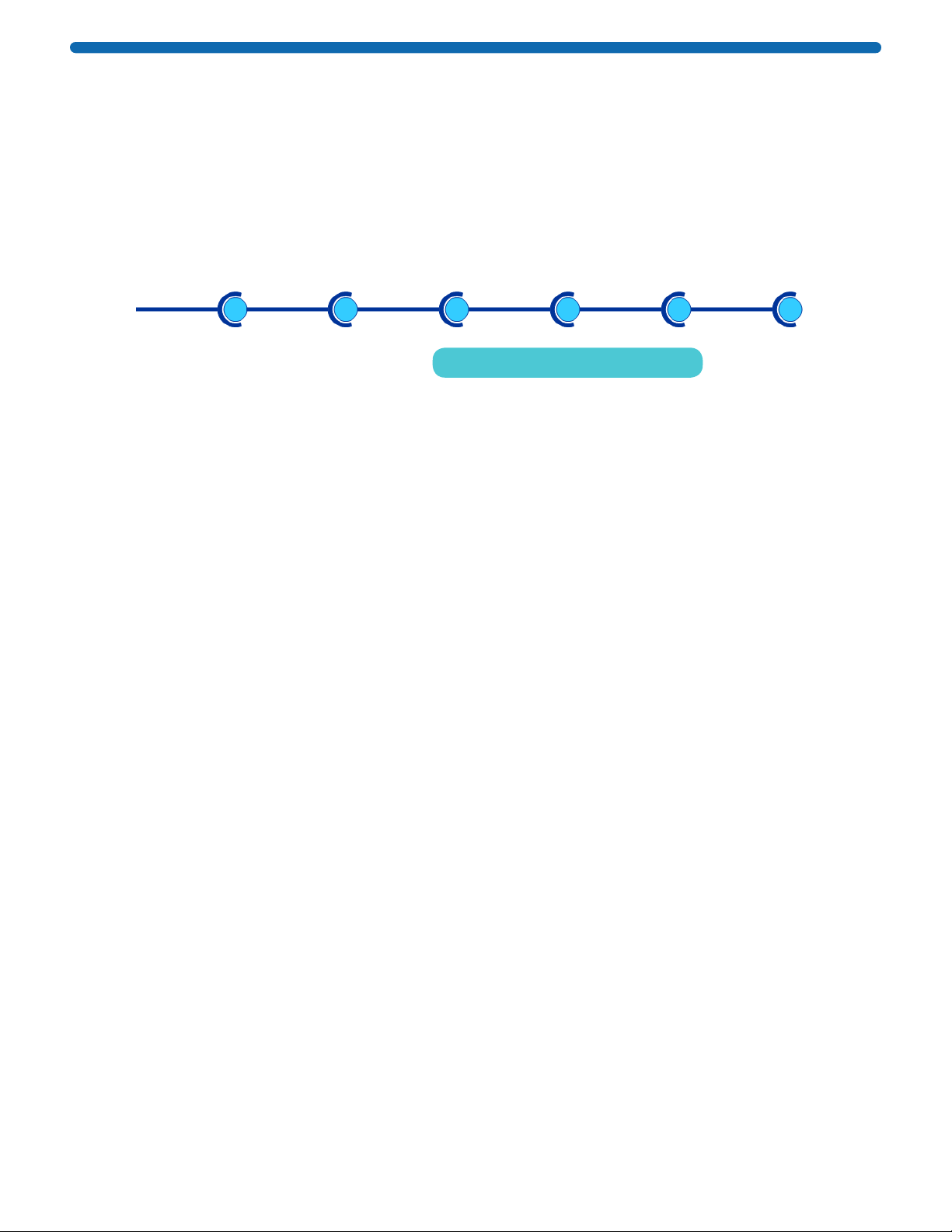
• Three removable blot holding frame sizes: MultiBlot, Mini, and Midi, to accommodate different blot sizes
• Disposable blot holders, sized for MultiBlot, Mini, and Midi frames
• Blot spacer required with first generation SNAP i.d.®system is now integrated into the new blot holder
• Extended and off-line blot processing options: frames have lids and can be removed from the base for
extended incubation (one hour to overnight), incubation in a shaker, or incubation at 4 °C
• Stackable frames so multiple blots can be processed at the same time
• 30-minute immunodetection with uniform signal across the blot
• Greater than 80% antibody recovery using the SNAP i.d.®2.0 Antibody Collection Trays
• Compatible with nitrocellulose and polyvinylidene fluoride (PVDF) membranes
• Works with the most blocking buffers and visualization methodologies (e.g. chemiluminescence, fluorescence,
or colorimetric)
The SNAP i.d.®2.0 Protein Detection System is intended for research use only. It is not for use in
diagnostic procedures.

00000825W REV 05/19 2 of 19
Parts and Functions of the SNAP i.d.®2.0 System
The SNAP i.d.®2.0 system consists of the following components:
A
B
C
D
E
J
I
H
M
L
K
F
Part Function Cat. No.
SNAP i.d.®2.0 Base Kit (includes the following components) SNAP2BASE
ABase Unit (1) Accepts frames and facilitates immunodetection
BTubing Assembly Kit (1) Connects system to vacuum source
CBlot roller (1) Eliminates air bubbles between the blot and blot holder
DRolling pad (1) Provides smooth surface for assembling blot
EWetting tray (2) Used for wetting out blot holder and blot
FAntibody Collection Tray (2) Collects antibody for recycling
GQuick Start Guide (not shown)
HSNAP i.d.®2.0 MultiBlot Holding
Frame and Lid
Accepts MultiBlot holders for blot incubation SNAP2FRMB01
ISNAP i.d.®2.0 Mini Blot Holding
Frame and Lid
Accepts Mini blot holders for blot incubation SNAP2FRMN01
SNAP2FRMN02
JSNAP i.d.®2.0 Midi Blot Holding
Frame and Lid
Accepts Midi blot holders for blot incubation SNAP2FRMD01
SNAP2FRMD02
KSNAP i.d.®2.0 MultiBlot Holder
(includes 2 MultiBlot well blanks)
Accepts up to 4.5 × 8.4 cm blots
Blank is used to block empty chamber when running only
one blot
SNAP2BHMB050
LSNAP i.d.®2.0 Mini Blot Holder Accepts up to 7.5 × 8.4 cm blots SNAP2BHMN0100
MSNAP i.d.®2.0 Midi Blot Holder Accepts up to 8.5 × 13.5 cm blots SNAP2BHMD0100

00000825W REV 05/19 3 of 19
Symbols Used in this User Guide
The following symbols are used throughout this user guide and/or on product labels, and the user shall abide by
indicated requirements:
Symbol Definition
YWarning alerts you to actions that
may cause personal injury or pose a
physical threat.
iRead the documentation
hCatalogue number
Symbol Definition
fSerial number
gLot number
MManufacturer
DDo not re-use
Materials Required but Not Supplied
• Vacuum source: Pump or other uniform vacuum source that can deliver a sustained minimum pressure of
410 millibar (12 inHg) and 34 L/min. Refer to Ordering Information for pump catalogue numbers.
NOTE: Our WP61 series Chemical Duty Pumps can be used, but may require longer processing times.
• One liter or larger vacuum flask with stopper (for waste collection). A Millex®-FA50 filter (or equivalent)
is recommended between the vacuum flask and the vacuum source to protect the vacuum source from
contamination.
• Vacuum tubing to connect vacuum flask to vacuum source
• Forceps
• Blocking reagent such as non-fat/low fat dry milk (0.5% or less), casein, bovine serum albumin (BSA) or other
commercially available blocking agents such as bløk®-CH buffer (see Table 5)
• Antibodies (monoclonal and/or polyclonal)
• Detection reagents
• Wash buffer: tris- or phosphate-buffered saline solution, pH 7.4, supplemented with Tween®20 surfactant (TBST
or PBST)
• Blot with transferred proteins
Blot Holder Size Maximum Blot Size (cm)
MultiBlot 4.5 × 8.4
Mini 7.5 × 8.4
Midi 8.5 × 13.5

00000825W REV 05/19 4 of 19
General Guidelines
• If proteins have been transferred to PVDF membrane that has been dried out, re-wet the blot with 100%
methanol, then rinse with distilled water prior assembly. If proteins have been transferred to nitrocellulose
membrane that has been dried out, re-wet the blot with distilled water.
• For most applications and for most antibodies, a 0.5% solution of non-fat/low fat dry milk provides sufficient
blocking of the membrane.
NOTE: Do not use dry milk concentrations higher than 0.5% because it can cause clogging of the blot holder
membrane. If using other blocking solutions, refer to Table 5 for compatibility and recommended concentration.
• Always use 0.1% Tween®20 surfactant in the washing, blocking, and antibody buffers. This will reduce the
surface tension of solutions and ensure even distribution of the antibody in the blot holder during incubation.
• Since immunodetection in the SNAP i.d.®2.0 system is performed in a short period of time, it is recommended
that primary and secondary antibody dilutions be prepared prior to starting the procedure.
• The total volume of diluted antibody required is 2.5 mL for the MultiBlot holder, 5 mL for the Mini blot holder,
and 10 mL for the Midi blot holder.
• Four washes of 30 mL each (15 mL for MultiBlot) are needed to ensure the complete wash of the blot after each
incubation step. Refer to Table 2 for details.
• Stripping blots in the SNAP i.d.
®
2.0 system is not recommended. Blots that have been stripped outside of the
system following a standard protocol can be reprobed in the SNAP i.d.
®
2.0 system starting with the blocking step.
• If stripping is not required (for example, if detecting with a different secondary antibody), blots can be reprobed
immediately in the SNAP i.d.®2.0 system after a quick wash.
00000825W REV 05/19 5 of 19
Optimization Guidelines
Antibodies
Optimization guidelines have been developed to enable users to convert the antibody concentrations used in
standard immunodetection assays into concentrations applicable to the SNAP i.d.®2.0 system. Most users will
be able to use the same amount of antibody, but in less volume at a higher concentration than for standard
immunodetection. However, when using the extended antibody protocol (page 12), it is possible to use the same
concentration, volume, and time for the primary antibody that is regularly used for standard immunodetection,
followed by the secondary antibody at a higher concentration for a shorter period of time.
Table 1. How to calculate the antibody concentration required for the SNAP i.d.®2.0 system based on the
concentration used for standard immunodetection
Standard
Immunodetection
SNAP i.d.®2.0 Immunodetection
MultiBlot Mini Blot Midi Blot
Ab stock concentration 1 mg/mL 1 mg/mL 1 mg/mL 1 mg/mL
Mass of antibody required 1 µg 0.25 µg 0.5 µg 1 µg
Volume of Ab used 30 mL 2.5 mL 5 mL 10 mL
Final Ab dilution 1 : 30,000 1 : 10,000 1 : 10,000 1 : 10,000
Antibody stock used 1 µL 0.25 µL 0.5 µL 1 µL
Because each antibody is unique, and the sensitivity of detection reagents varies, it may be necessary to adjust
the antibody or antigen concentration, the type or sensitivity of the detection reagent used, or the blot X-ray
exposure time. Please note that this guideline is intended as a starting point to develop the final antibody
concentration for the desired performance.
Table 2. Blocking, Antibody, and Wash Recommended Volumes
SNAP i.d.®2.0
MultiBlot Frame
SNAP i.d.®2.0
Mini Frame
SNAP i.d.®2.0
Midi Frame
Blocking solution volume 15 mL per well 30 mL 30 mL
Antibody volume 2.5 mL per well 5 mL 10 mL
Wash buffer* volume 4 × 15 mL each per well 4 × 30 mL each 4 × 30 mL each
* Tris- or phosphate-buffered saline solutions, supplemented with 0.1% Tween®20 surfactant
When calculating the amount of antibody required for immunodetection, three components are critical for
successful detection of the protein and a good quality blot (low background, high signal):
• Type of sample and concentration loaded on the gel
• Primary and secondary antibody concentration
• Type and sensitivity of the detection reagent
All of the antibodies in the following list were tested using Luminata™ Forte chemiluminescence detection reagent.
For the SNAP i.d.®2.0 system, the antibody concentration is higher than in standard immunodetection, but in less
volume.

00000825W REV 05/19 6 of 19
Table 3. Examples of Primary Antibody Dilution in Standard Immunodetection vs.
SNAP i.d.®2.0 Immunodetection
NOTE: All antibodies were tested using 0.5% NFDM as the blocking reagent and Luminata™ Forte Western HRP
Substrate as the chemiluminescent reagent.
Primary AntibodyaHostbCat. No. Protein MW
Standard
Ab dilution
SNAP i.d.®2.0
Ab dilution
Anti-Akt1/PKBα Rb 05-796 60 1 : 1,200–1 : 2,000 1 : 400
Anti-CREB Rb 06-863 43 1 : 600–1 : 1,000 1 : 200
Anti-Caspase-3 Rb AB3623 17 1 : 1,200–1 : 2,000 1 : 400
Anti-Cyclin D1 Rb 04-1151 36 1 : 600–1 : 1,000 1 : 200
Anti-EGFR M 05-104 170 1 : 600–1 : 1,000 1 : 200
Anti-MAP Kinase 1/2 ErK1/2 Rb 06-182 44-42 1 : 1,500–1 : 2,500 1 : 500
Anti-erbB2 M 04-291 185 1 : 600–1 : 1,000 1 : 200
Anti-GAPDH M MAB374 38 1 : 30,000–1 : 50,000 1 : 10,000
Anti-GST M1 (mu) Rb ABN19 26 1 : 600–1 : 1,000 1 : 200
Anti-GFAP M MAB3402 50 1 : 1,200–1 : 2,000 1 : 400
Anti-Glutamate receptor 1 Rb AB1504 100 1 : 1,200–1 : 2,000 1 : 400
Anti-Huntington protein M MAB2166 350-400 1 : 1,200–1 : 2,000 1 : 400
Anti-Integrin α5 Rb AB1928 114 1 : 3,000–1 : 5,000 1 : 1,000
Anti-MGluR5 Rb AB5675 132 1 : 600–1 : 1,000 1 : 200
Anti-NFkB p52 Rb 06-413 100 & 52 1 : 1,200–1 : 2,000 1 : 400
Anti-NMDAR1 Rb AB9864 120 1 : 1,200–1 : 2,000 1 : 400
Anti-P53 (N-term) Rb 04-1083 53 1 : 600–1 : 1,000 1 : 200
Anti-pan-Cadherin Rb ABT35 120-130 1 : 1,200–1 : 2,000 1 : 400
Anti-PP2A M 05-421 36 1 : 3,000–1 : 5,000 1 : 1,000
Anti-PTEN M 04-035 55 1 : 600–1 : 1,000 1 : 200
Anti-Pyk2 Rb 06-559 114 1 : 600–1 : 1,000 1 : 200
Anti-Ras M 05-516 21 1 : 1,500–1 : 2,500 1 : 500
Anti-Rac1 M 05-389 21 1 : 600–1 : 1,000 1 : 200
Anti-STAT1 M 05-987 91 1 : 300–1 : 500 1 : 100
Anti-Tau1 M MAB3420 52-68 1 : 1,200–1 : 2,000 1 : 400
Anti-β-Tubulin M MAB3408 50 1 : 3,000–1 : 5,000 1 : 1,000
aGo to www.sigmaaldrich.com/antibodies for details
bM = Mouse, Rb = Rabbit
Table 4. Examples of Secondary Antibody Dilution in Standard Immunodetection
vs. SNAP i.d.®2.0 Immunodetection
Secondary AntibodyaHostbCat. No.
Standard
Ab dilution
SNAP i.d.®2.0
Ab dilution
Anti-Mouse IgG Gt AP124P 1 : 50,000 1 : 10,000
Anti-Rabbit IgG Gt AP132P 1 : 200,000 1 : 30,000
Anti-Sheep IgG Rb AP147P 1 : 50,000 1 : 20,000
Anti-Goat IgG Rb AP106P 1 : 80,000 1 : 10,000
a Horseradish peroxidase conjugate
b Gt = Goat, Rb = Rabbit

00000825W REV 05/19 7 of 19
Blot Blocking
• The SNAP i.d.®2.0 system is compatible with most commonly used blocking agents including non-fat/low fat dry
milk, bovine serum albumin (BSA) and casein. Many other commercially available blockers are also compatible
(refer to Table 5).
• In order to ensure optimal flow through the blot holder, it is essential that blocking solutions be completely
solubilized and free of all particulate matter. In some cases, it may be necessary to reduce the concentration of
the blocking agent to achieve the required flow.
• The use of non-fat/low fat dry milk at concentrations higher than 0.5% is not recommended.
• Blocking agents, should be prepared in tris- or phosphate-buffered saline solution containing 0.1% Tween®20
surfactant, to reduce surface tension and ensure even distribution of blocking agent across the blot holder
surface.
• To ensure even distribution of the antibody in the incubation step, dilute the antibody in blocking solution that
contains Tween®20 surfactant.
Table 5. Compatibility with Blocking Reagents
Blocker Compatible
Recommended
Concentration
Non-fat/low fat dry milk (NFDM) Yes, ≤ 0.5% 0.5%
Immunoblot Blocking Reagent, NFDM (cat. no. 20-200) Yes, ≤ 0.5% 0.5%
BLOT-QuickBlocker™ Reagent (cat. no. WB57-175GM) Yes 0.5%
bløk®-PO Buffer (cat. no. WBAVDP001) Yes Undiluted
bløk®-FL Buffer (cat. no. WBAVDFL01) Yes Undiluted
bløk®-CH Buffer (cat. no. WBAVDCH01) Yes Undiluted
N-Z-Amine®AS (Fluka®)Yes, ≤ 5% 1%
Probumin®Bovine Serum Albumin (BSA) (cat. no. 820473) Yes, ≤ 5% 1%
5% Alkali-soluble Casein (Novagen®cat. no. 70955) Yes 1%
SEA BLOCK Blocking Buffer (Pierce®) Yes Undiluted
SuperBlock®Blocking Buffer with Tween®20 surfactant (Pierce®) Yes Undiluted
LI-COR®Odyssey®Blocking Buffer Yes Undiluted
PVP-40 (Polyvinylpyrrolidone) Yes, ≤ 1% 1%
Gelatin No N/A
Immobilon®Signal Enhancer (cat. no. WBSH0500) Yes Undiluted
00000825W REV 05/19 8 of 19
How to Use the SNAP i.d.®2.0 System
System Setup
1. Place the SNAP i.d.®2.0 base on a level bench top.
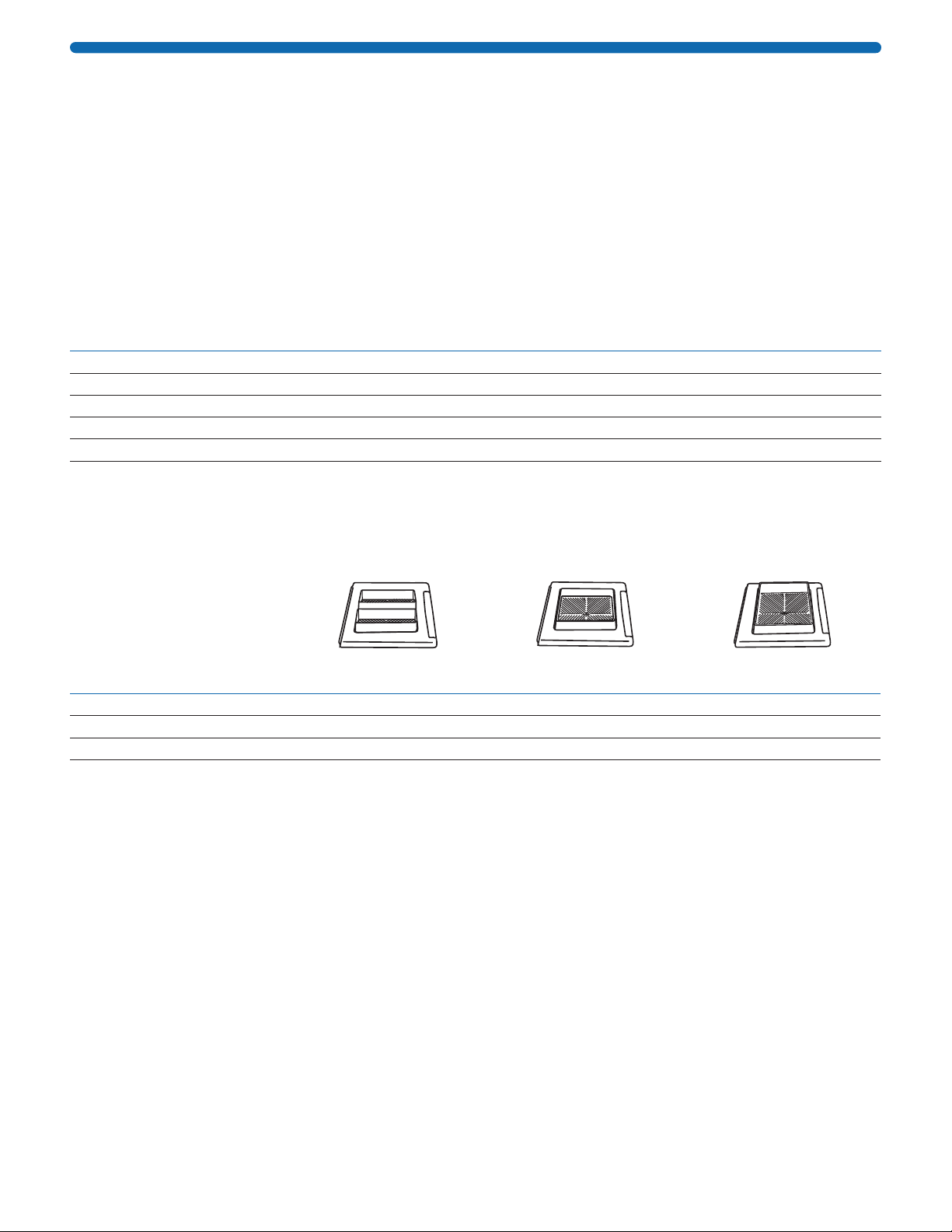
2. Attach the vacuum tubing to the back of the system by pushing the coupling insert on the end of the tubing
into the quick disconnect fitting at the back of the system base until it clicks.
Push tab up to
insert or release
coupling To vacuum
NOTE: To disconnect the tubing, push the tab below the tubing connector up with the index finger and pull
tubing out.
3. Connect the other end of the tubing to a vacuum source. Use a one-liter vacuum flask as a trap and a
Millex®-FA50 filter (cat. no. SLFA05010) to protect the vacuum source from contamination, as shown below.
To SNAP i.d.®
2.0 System
Tra p Vacuum source
Vacuum
protection filter
NOTE: Any vacuum source that can deliver 410 millibar (12 inHg) and 34 L/min is sufficient. If the vacuum
source operates at higher than 410 millibar, the SNAP i.d.®2.0 system will automatically regulate the vacuum
pressure.
If the vacuum source is insufficient, the flow rate through the system may be inconsistent, resulting in longer
processing times and/or poor antibody recovery.

00000825W REV 05/19 9 of 19
Blot Assembly and General Protocol
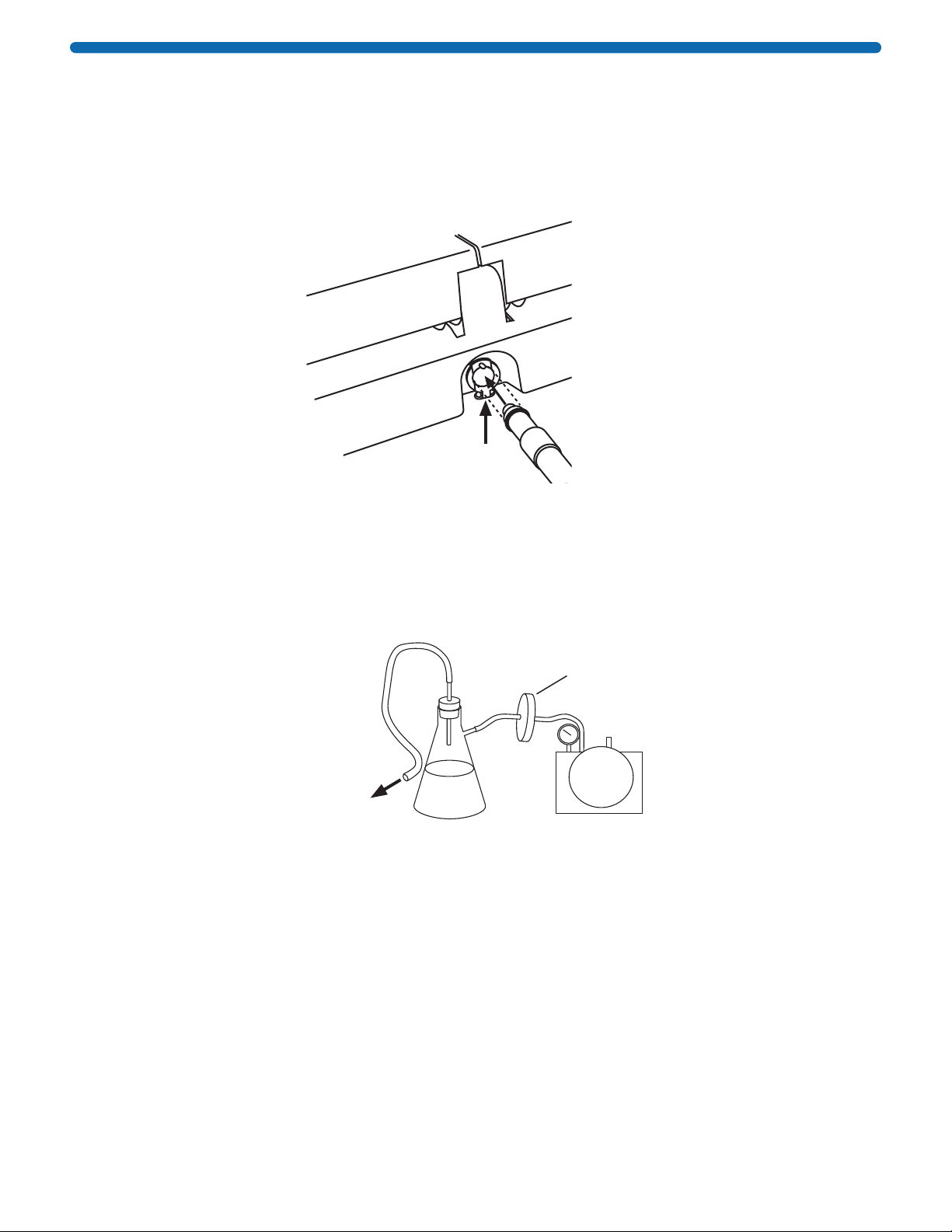
1. Hold the blot holder by the support layer (blue edges) and wet
the membrane layer (white) with distilled water in the wetting
tray provided. Do not wet the support layer. Place the wetted
blot holder on the rolling pad.
2. If required, pre-wet the blot in methanol and water, then place it
in the center of the blot holder with the protein side down.
NOTE: Blot should not exceed size specified in the Materials
Required section.
3. Roll the blot gently to remove air bubbles, then close the blot
holder and roll one more time.
4. Open the blot holding frame, flip the blot holder so that it is
protein side up, then place it inside the frame. A notch in the
blot holder ensures correct placement in the frame.
NOTE: If running only one MultiBlot in the frame, place the well
blank card in the second well.
5. Close and lock the frame. Add 30 mL of blocking solution
(15 mL for MultiBlot). Press the frame down and turn the system
knob to apply vacuum. When frame is completely empty,
TURN VACUUM OFF.
NOTE: If using antibody recovery trays, insert trays after step 5.
For details, refer to Antibody Recovery section.
Alignment
notch

00000825W REV 05/19 10 of 19
6. Apply appropriate volume of primary antibody across the surface
of the blot holder (2.5 mL for MultiBlot, 5 mL for Mini blot, or
10 mL for Midi blot).
7. Incubate for 10 minutes at room temperature. Solution will be
absorbed into the blot holder and surface may appear dry.
IMPORTANT: Do not apply vacuum until after the
10-minute incubation.
8. Press the frame down and apply vacuum. Wait 5–8 seconds until
the frame is completely empty.
9. With vacuum running continuously, add 30 mL of wash buffer
(15 mL for MultiBlot). Repeat the washing step 3 more times
(total of 4 washes). When frame is completely empty, TURN
VACUUM OFF.
10. Apply appropriate volume of secondary antibody across the surface of the blot holder (2.5 mL for MultiBlot,
5 mL for Mini blot, or 10 mL for Midi blot). Incubate for 10 minutes at room temperature with vacuum off.
Again, solution will be absorbed into the blot holder and surface may appear dry.
IMPORTANT: Do not apply vacuum until after the 10-minute incubation.
11. Press frame down and apply vacuum. Wait 5–8 seconds until frame is completely empty. With vacuum running
continuously, add 30 mL of wash buffer (15 mL for MultiBlot). Repeat the washing step 3 more times (total of
4 washes).
12. Turn vacuum off and remove blot holder from frame. Remove blot from blot holder and incubate with the
appropriate detection reagent. If the MultiBlot well blank was used, remove and clean.
4X
00000825W REV 05/19 11 of 19
Extended Primary Antibody Incubation: One Hour to Overnight
1. Perform steps 1 through 5 of the General Protocol.
2. Add 2.5 mL (for MultiBlot), 5 mL (for Mini blot), or 10 mL (for Midi blot) of 1X primary antibody (concentration
normally used during standard immunodetection).
3. Cover the blot holding frame with the lid. If desired, remove it from the base and incubate with constant
shaking. If overnight incubation is required, refrigeration is recommended. Although the surface of the blot
holder may look partially dry, the blot will not dry out, since the solution is contained in the frame.
NOTE: If processing more than one blot for extended period of time, the blot holding frames can be stacked
to reduce the working space.
Optional: If recovering primary antibody, place antibody recovery tray(s) into base before proceeding to step 4.
4. After the extended incubation period, place the frame back on the base, remove the lid, and follow steps 8
through 12 of the General Protocol.
NOTE: Extended incubation periods for the secondary antibody are not required for the SNAP i.d.®2.0
System. A 5X concentration of secondary antibody for 10 minutes is recommended.
Antibody Recovery
1. Perform steps 1 through 5 of the General Protocol, but increase the vacuum time to 2 minutes to ensure that
all the blocking solution has been removed from the grooves and channels of the frame.
2. Remove the blot holding frame from the base and wipe any residual liquid from the bottom of the frame with a
paper towel.
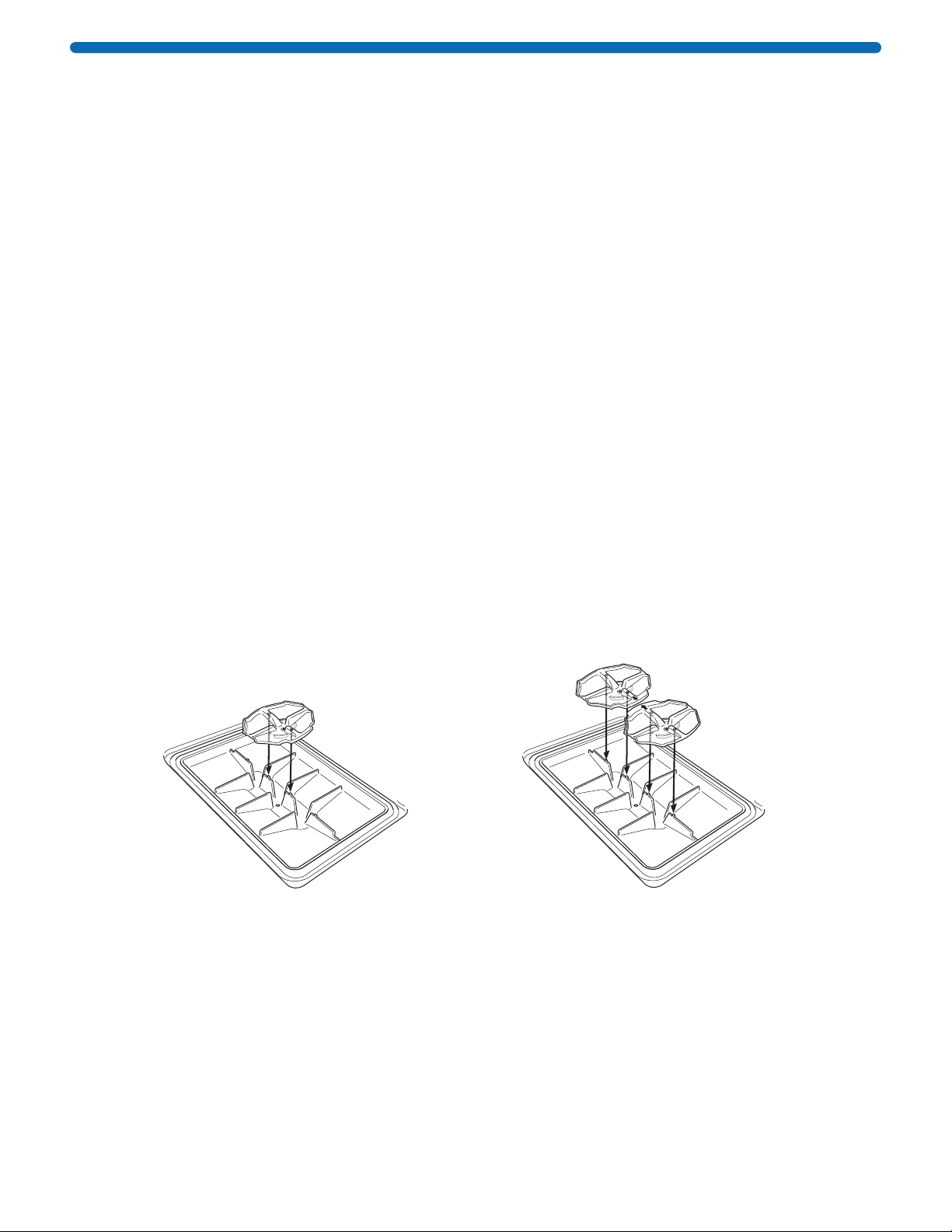
3. Place the antibody collection tray(s) into the base, making sure that the positioning holes in the antibody
collection tray line up with the positioning pins in the base.
NOTE: For Mini and Midi frames, position a single collection tray in the center of the base. For the MultiBlot
frame, position two collection trays as shown in the diagram.
When running a single blot in the MultiBlot frame, place a well blank card in the empty well and position the
collection tray below the well with the blot.
Align positioning
holes on tray with
pins on base.
Antibody
collection tray
tabs face towards
one another
SNAP i.d.®2.0 Mini and Midi Frames SNAP i.d.®2.0 MultiBlot Frame
4. Place the blot holding frame back into position on the base.
5. Apply primary antibody and incubate as indicated in steps 6 and 7 of the General Protocol.
6. After the 10-minute incubation, turn the vacuum on and wait one minute to ensure that all of the antibody has
been collected.
NOTE: When processing two frames at the same time, apply vacuum first to one side, then to the other. This
ensures full vacuum force on each frame and improves volume recovery.
7. Turn the vacuum off and remove the frame. Remove the antibody collection tray.
8. Transfer the antibody to a suitable container for storage or analysis.
9. Place the blot holding frame back into position and continue with step 9 of the General Protocol.

00000825W REV 05/19 12 of 19
Proof of Performance
Figure 1. Immunodetection of β-Tubulin:
SNAP i.d.®2.0 System vs. SNAP i.d.®System (first generation) vs. Standard Immunodetection
Blocking 0.5% NFDM for 20 sec 0.5% NFDM for 20 sec 0.5% NFDM for 1 hr
Primary Ab β-Tubulin 1 : 1,000 for 10 min β-Tubulin 1 : 1,000 for 10 min β-Tubulin 1 : 5,000 for 1 hr
Secondary Ab Gt × M 1 :10,000 for 10 min Gt × M 1 :10,000 for 10 min Gt × M 1 :50,000 for 1 hr
Total Time 30 min 30 min 3 hr 30 min
b. SNAP i.d.®Protein Detection
System First generation, single well
1 2 3 4 5 1 2 3 4 5
3T3 Anisomycin 3T3 PDGF
188
98
62
49
38
28
18
14
6
a. SNAP i.d.®2.0 Protein Detection
System Midi Blot
3T3 Control 3T3 Anisomycin 3T3 PDGF
188
98
62
49
38
28
18
14
6
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
MW
c. Standard
Immunodetection
188
98
62
49
38
28
18
14
6
3T3 Control 3T3 Anisomycin 3T3 PDGF
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
Control, anisomycin-treated, and PDGF-treated 3T3 mouse fibroblast cell lysates
(10-0.63 µg) were transferred to Immobilon®-P membrane. Blots were blocked
with 0.5% non-fat dry milk (NFDM) prepared in tris-buffered saline supplemented
with 0.1% Tween® 20 surfactant (TBST), and probed with primary anti-β-Tubulin
(MAB3408) and secondary HRP-conjugated goat anti-mouse (AP124P) at the
conditions indicated above. Blots were exposed to X-ray film for one minute.
Lane Concentration (µg)
1 10
2 5
3 2.5
4 1.25
5 0.63
Figure 2. Immunodetection of erbB2:
SNAP i.d.®2.0 System vs. SNAP i.d.®System (first generation) vs. Standard Immunodetection
Blocking 0.5% NFDM for 20 sec 0.5% NFDM for 20 sec 0.5% NFDM for 1 hr
Primary Ab erbB2 1 :500 for 10 min erbB2 1: 500 for 10 min erbB2 1 :2,500 for 1 hr
Secondary Ab Gt × M 1 :10,000 for 10 min Gt × M 1 :10,000 for 10 min Gt × M 1 : 50,000 for 1 hr
Total Time 30 min 30 min 3 hr 30 min
b. SNAP i.d.®Protein Detection
System First generation, single well
188
98
62
49
38
28
18
14
6
123456789
c. Standard
Immunodetection
188
98
62
49
38
28
18
14
6
123456789
a. SNAP i.d.®2.0 Protein
Detection System Mini Blot
123456789
188
98
62
49
38
28
18
14
6
MW
A431 EGF stimulated cell lysate (20 to 0.08 µg) was
transferred to Immobilon®-P membrane. Blots were
blocked with 0.5% NFDM prepared in TBST, and probed
with primary anti-erbB2 (04-291) and secondary HRP-
conjugated goat anti-mouse (AP124P) at the conditions
indicated above. Blots were exposed to X-ray film for
5 minutes.
Lane Concentration (µg)
1 20
2 10
3 5
4 2.5
5 1.25
Lane Concentration (µg)
6 0.63
7 .31
8 .16
9 .08

00000825W REV 05/19 13 of 19
Figure 3. Immunodetection of Tau-1 protein:
SNAP i.d.®2.0 System (MultiBlot, Midi, and Mini) vs. Standard Immunodetection
a. SNAP i.d.®2.0 MultiBlot
b. SNAP i.d.®2.0
Mini Blot
c. SNAP i.d.®2.0
Midi Blot
d. Standard
Immunodetection
MW
Alzheimer’s brain Healthy brain
188
98
62
49
38
28
14
6
3
1 2 3 4 5 1 2 3 4 5
Alzheimer’s brain Healthy brain
188
98
62
49
38
28
14
6
3
1 2 3 4 5 1 2 3 4 5
Alzheimer’s brain Healthy brain
188
98
62
49
38
28
14
6
3
1 2 3 4 5 6 7 8 9 1 2 3 4 5 6 7 8 9
Alzheimer’s brain Healthy brain
188
98
62
49
38
28
14
6
3
1 2 3 4 5 1 2 3 4 5
Blocking 0.5% NFDM for 20 sec 0.5% NFDM for 20 sec 0.5% NFDM for 20 sec 0.5% NFDM for 1 hr
Primary Ab Anti-Tau1 1:1,000 for 10 min Anti-Tau1 1 : 1,000 for 10 min Anti-Tau1 1 : 1,000 for 10 min Anti-Tau1 1 :5,000 for 1 hr
Secondary Ab Gt × M 1:10,000 for 10 min Gt × M 1 :10,000 for 10 min Gt × M 1 :10,000 for 10 min Gt × M 1 :50,000 for 1 hr
Total Time < 30 min < 30 min < 30 min 3 hr 30 min
Human brain samples from an Alzheimer’s patient and
from a healthy donor were lysed in Cytobuster™ Protein
Extraction Reagent (cat. no. 71009). Samples were serially
diluted and separated by SDS gel electrophoresis. Gels
were transferred to Immobilon®-P membrane. Blots were
processed in the SNAP i.d.®2.0 system using MultiBlot,
Mini, and Midi frames with their corresponding blot holders.
A control blot was processed by standard immunodetection.
All blots were blocked with 0.5% NFDM and probed with
primary anti-Tau1 (cat. no. MAB3420) and secondary HRP-
conjugated goat anti-mouse (AP124P) at the conditions
indicated above. Blots were incubated with Luminata™ Forte
HRP substrate and exposed to X-ray film for 15 minutes.
MultiBlot, Mini
Blot, and Standard
Immunodetection
Lane Concentration (µg)
120
210
35
42.5
51.25
Midi Blot
Lane Concentration (µg)
120
210
35
42.5
51.25
60.63
7.31
8.16
9.08
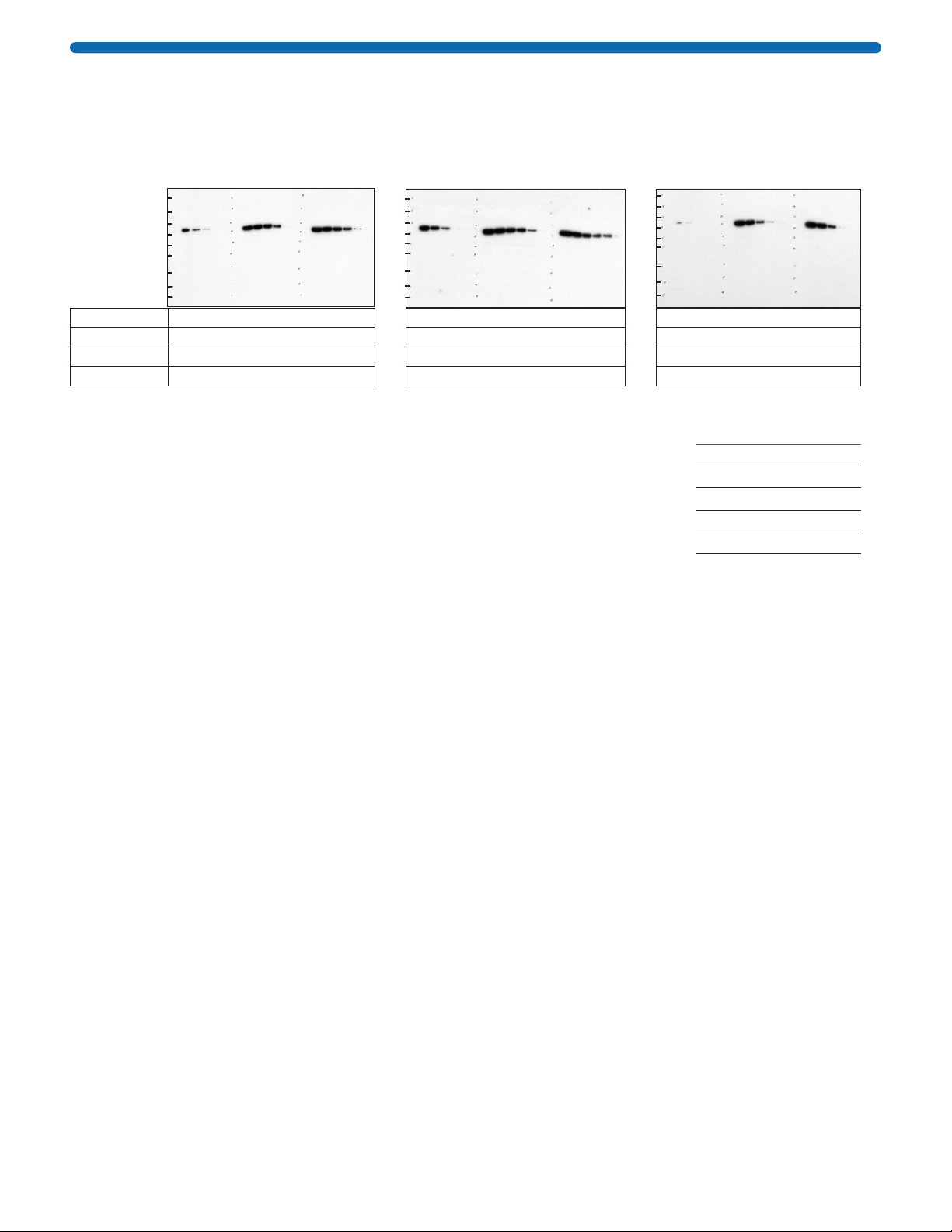
Figure 4. Extended Incubation (Overnight):
SNAP i.d.®2.0 System vs. Standard Immunodetection
Blocking 0.5% NFDM for 20 sec 0.5% NFDM for 1 hr
Primary Ab ErK1/2 1:2,500 for 16 hr ErK1/2 1: 2,500 for 16 hr
Secondary Ab Gt × Rb 1 :30,000 for 10 min Gt × Rb 1 : 150,000 for 1 hr
Total Time 16 hr 20 min 18 hr 30 min
a. SNAP i.d.®2.0 Protein Detection
System Midi Blot
1 2 3 4 5 6 7 8 9 1 2 3 4 5 6 7 8 9
62
49
38
28
18
14
6
3
188
MW A431 cell lysate Rat liver lysate
b. Standard
Immunodetection
1 2 3 4 5 6 7 8 9 1 2 3 4 5 6 7 8 9
62
49
38
28
18
14
6
3
188 A431 cell lysate Rat liver lysate
A431 cell lysate and rat liver lysate (12 to 3 µg) were transferred to Immobilon®-P
membrane. Both blots were blocked with 0.5% NFDM prepared in TBST. The
SNAP i.d.®2.0 Midi blot was blocked for 20 seconds and the standard blot was
blocked for 1 hour. Both blots were incubated overnight with the same dilution of
MAP-Kinase 1/2 (ErK1/2), however, for the secondary antibody, HRP-conjugated
goat anti-rabbit (AP132P), the SNAP i.d.®2.0 blot was incubated for 10 minutes
and the standard blot was incubated for 1 hour after 3 washes in TBST.
Lane Concentration (µg)
1 12
2 6
3 3
4 12
5 6
6 3
7 12
8 6
9 3
00000825W REV 05/19 14 of 19
Figure 5. Extended Incubation (1 Hour):
SNAP i.d.®2.0 System 1 Hour Protocol vs. SNAP i.d.®2.0 System Standard Protocol vs.
Standard Immunodetection
Blocking 0.5% NFDM for 20 sec 0.5% NFDM for 20 sec 0.5% NFDM for 1 hr
Primary Ab β-Tubulin 1 : 5,000 for 1 hr β-Tubulin 1 : 1,000 for 10 min β-Tubulin 1 : 5,000 for 1 hr
Secondary Ab Gt × M 1 :10,000 for 10 min Gt × M 1 :10,000 for 10 min Gt × M 1 :50,000 for 1 hr
Total Time 1 hr 15 min 30 min 3 hr 30 min
188
98
62
49
38
28
18
14
6
3T3 Control 3T3 Anisomycin 3T3 PDGF
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
a. SNAP i.d.®2.0 Protein Detection
System Midi Blot (1 hr protocol)
b. SNAP i.d.®2.0 Protein Detection
System Midi Blot (Standard protocol)
3T3 Control 3T3 Anisomycin 3T3 PDGF
188
98
62
49
38
28
18
14
6
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
MW
c. Standard
Immunodetection
188
98
62
49
38
28
18
14
6
1 2 3 4 5 1 2 3 4 5 1 2 3 4 5
3T3 Control 3T3 Anisomycin 3T3 PDGF
Control, anisomycin-treated, and PDGF-treated 3T3 mouse fibroblast cell lysates
(10-0.63 µg) were transferred to Immobilon®-P membrane. Blots were blocked
with 0.5% non-fat dry milk (NFDM) prepared in tris-buffered saline supplemented
with 0.1% Tween® 20 surfactant (TBST), and probed with primary anti-β-Tubulin
(MAB3408) and secondary HRP-conjugated goat anti-mouse (AP124P) at the
conditions indicated above. Blots were exposed to X-ray film for one minute.
Lane
Concentration
(µg)
1 10
2 5
3 2.5
4 1.25
5 0.63
Maintaining the SNAP i.d.®2.0 System
Cleaning Protocol
The SNAP i.d.®2.0 system should be cleaned after each use. To remove salts or contaminants from the base
and tubing, turn the vacuum on and flush distilled water through the system.
NOTE: Do not autoclave the SNAP i.d.®2.0 system.
Frames can be disassembled and washed with a mild detergent, then rinsed thoroughly with distilled water and
air dried.
To disassemble the frame, orient it with the blue clamp on the right side. Unclamp the frame, and using both
hands, open it like a book, while gently pushing the left half down and the right half up. When the frame is
almost completely open, the two halves will slide apart. Take care not to lose the small O-ring located on one
of the center pins.
To reassemble the frame, reverse the procedure above. It may take slightly more force to engage the two
halves, since the O-ring is being compressed.
NOTE: The purpose of this O-ring is to keep the frame cover opened when placing the blot in the frame. The
frame is fully functional without the O-ring.
Component Re-use
Blot holders and antibody collection trays are single-use only. Re-use increases the risk of blot holder clogging
and uneven signal across the blot, as well as sample cross-contamination.
Refer to applicable international, federal, state, and local regulations for appropriate disposal.
00000825W REV 05/19 15 of 19
Troubleshooting
Symptom Cause Corrective Action
Vacuum control knobs stick Inadequate cleaning Flush system with distilled water.
Blot holders do not empty
or empty slowly when
vacuum is applied
Inadequate vacuum Make sure tubing connection between system and vacuum
source is secure.
MultiBlot frame was used to
process a single blot, and well
blank card was not placed in
empty well
When running a single blot in the MultiBlot frame, place well
blank card in empty well.
Inadequate cleaning Make sure all system gaskets and valve at center of frame are
clean and free of debris or salt.
Empty the vacuum flask and change the in-line Millex
®
-FA filter.
Blot holder may be clogged due to:
Concentration of
non-fat/low fat dry
milk too high
Use 0.2–0.5% dry milk in buffer supplemented with
0.1% Tween®20 surfactant, with a new blot holder.
Incompatible blocking solution Change to a different blocking solution with a new blot holder.
Re-use of blot holder Blot holders are intended for single use. Do not re-use.
Low signal or signal lower
than in standard
immunodetection
Primary and/or secondary
antibody concentration too low
Increase the antibody concentration 5 to 8-fold.
Blot upside down Mark the protein side of the blot and make sure that this side
is facing the membrane side of the blot holder.
Detection reagent not sensitive
enough
Change to a higher sensitivity detection reagent such as
Luminata™ Forte Western HRP Substrate.
High background Inadequate blocking Change to different blocking solution.
Blot holder not wet enough Pre-wet the blot holder before assembly.
Blocking solution may have
degraded
Always prepare fresh 0.5% non-fat/low fat dry milk.
Antibody concentration too high Decrease the concentration of antibody.
Blot holder was placed upside
down in the frame
Place blot holder in frame protein side up.
Inadequate washing Run at least 4 washes of 30 mL each.
Make sure vacuum is running continuously while adding wash
buffer.
Inconsistent signal across
the blot
System not level Make sure system is on a flat, level surface.
Antibody not applied evenly
across entire surface of
blot holder
Supplement blocking solution and antibody diluent with
0.1% Tween®20 surfactant.
Use a small volume pipette (e.g., 5 mL) and slowly distribute
the antibody across the entire blot.
Apply at least 2.5 mL (for MultiBlot), 5 mL (for Mini blot), or
10 mL (for Midi blot) of antibody.
Low antibody volume
recovery
Antibody recovery trays not
placed correctly in base
Make sure that the holes in the antibody recovery tray align
with the pins in the base.
Two frames processed
simultaneously
Apply vacuum first to one frame, then to the other, to ensure
full vacuum force on each frame.
MultiBlot frame was used to
process a single blot, and well
blank card was not placed in
empty well
When running a single blot in the MultiBlot frame, place well
blank card in empty well.

00000825W REV 05/19 16 of 19
Specifications
Dimensions
Base (length × width × height) 40.6 cm (16 in.) × 32.4 cm (12.75 in.) × 8.9 cm (3.5 in.)
Weight (approximate) 1.5 kg (3.3 lb)
MultiBlot frame with lid (length × width × height) 19.7 cm (7.75 in.) × 14.7 cm (5.8 in.) × 3.6 cm (1.4 in.)
MultiBlot holder 11.4 cm (4.5 in.) × 6.4 cm (2.5 in.)x
Mini frame with lid (length × width × height) 19.7 cm (7.75 in.) × 14.7 cm (5.8 in.) × 3.6 cm (1.4 in.)
Mini blot holder 12.7 cm (5.0 in.) × 9.1 cm (3.6 in.)
Midi frame with lid (length × width × height) 19.7 cm (7.75 in.) × 14.7 cm (5.8 in.) × 3.6 cm (1.4 in.)
Midi blot holder 17.8 cm (7.0 in.) × 10.2 cm (4.0 in.)
Antibody tray (length × width × height) 6.4 cm (2.5 in.) × 5.8 cm (2.3 in.) × 1.9 cm (0.75 in.)
Materials of Construction
System
Base and top Acrylonitrile butadiene styrene (ABS)
Gaskets Silicone
Tubing Silicone
Tubing fittings Polypropylene or acetal with ethylene propylene diene monomer (EPDM)
or Buna-N seals
Frames
Top and bottom ABS
Latch ABS
Gaskets Silicone
Valve EPDM
Lid Polystyrene
Blot holder
Membrane layer PVDF with high impact polystyrene (HIPS)
Support layer Polypropylene with patapar
Accessories
Roller Acetal and stainless steel
Rolling pad Polyester, HIPS, and acrylic
Antibody collection tray Styrene butadiene copolymer
Wetting tray Polyethylene terephthalate glycol (PETG)
Chemical Compatibility
The SNAP i.d.®2.0 Protein Detection System is compatible with aqueous solutions and dilute acids and bases.
Do not expose to organic solvents.
Storage
Store all components at room temperature.

00000825W REV 05/19 17 of 19
Ordering Information
Purchase products online at www.sigmaaldrich.com/products.
Product Description Cat. No. Qty/Pk
Base System
SNAP i.d.®2.0 Base SNAP2BASE 1
Base unit (1)
Tubing assembly kit (1)
Blot roller (1)
Rolling pad (1)
Wetting trays (2)
Antibody collection trays (2)
Quick-Start Guide (1)
Components for Western Blotting Procedures
SNAP i.d.®2.0 MultiBlot Holding Frame SNAP2FRMB01 1
MultiBlot frame with lid (1)
MultiBlot holders (2)
SNAP i.d.®2.0 Mini Blot Holding Frame (single pack) SNAP2FRMN01 1
Mini frame with lid (1)
Mini blot holders (2)
SNAP i.d.®2.0 Mini Blot Holding Frames (double pack) SNAP2FRMN02 1
Mini frame with lid (2)
Mini blot holders (4)
SNAP i.d.®2.0 Midi Blot Holding Frame (single pack) SNAP2FRMD01 1
Midi frame with lid (1)
Midi blot holders (2)
SNAP i.d.®2.0 Midi Blot Holding Frames (double pack) SNAP2FRMD02 1
Midi frame with lid (2)
Midi blot holders (4)
SNAP i.d.®2.0 MultiBlot Holders (includes 2 well blanks) SNAP2BHMB050 50
SNAP i.d.®2.0 Mini Blot Holders SNAP2BHMN0100 100
SNAP i.d.®2.0 Midi Blot Holders SNAP2BHMD0100 100
SNAP i.d.®2.0 Antibody Collection Tray SNAPABTR 20
SNAP i.d.®Blot Roller SNAP2RL 1
Blotting Membranes
Immobilon®-E PVDF, 0.45 µm, 7 cm × 8.4 cm sheets IEVH07850 50
Immobilon®-E PVDF, 0.45 µm, 8.5 cm × 10 m roll IEVH85R 1
Immobilon®-E PVDF, 0.45 µm, 26.5 cm × 1.875 m roll IEVH00005 1
Immobilon®-E PVDF, 0.45 µm, Blotting Sandwich, 7 cm × 8.4 cm IESN07852 20
Immobilon
®
-E PVDF
, 0.45 µm
, Blotting Sandwich, 8.5 cm × 13.5 cm
IESN08132 10
Immobilon®-P PVDF, 0.45 µm, 7 cm × 8.4 cm sheet IPVH07850 50
Immobilon®-P PVDF, 0.45 µm, 8.5 cm × 13.5 cm sheet IPVH08130 10
Immobilon®-P PVDF, 0.45 µm, 8.5 cm × 10 m roll IPVH85R 1
Immobilon®-P PVDF, 0.45 µm, 26.5 cm × 375 cm roll IPVH00010 1
Immobilon®-FL PVDF, 0.45 µm, 7 cm × 8.4 cm sheet IPFL07810 10
Immobilon®-FL PVDF, 0.45 µm, 8.5 cm × 10 m roll IPFL85R 1
Immobilon®-FL PVDF, 0.45 µm, 26.5 cm × 375 cm roll IPFL00010 1
Immobilon®-PSQ PVDF, 0.2 µm, 7 cm × 8.4 cm sheet ISEQ07850 50
Immobilon®-PSQ PVDF, 0.2 µm, 8.5 cm × 10 m roll ISEQ85R 1

00000825W REV 05/19 18 of 19
Product Description Cat. No. Qty/Pk
Reagents for Western Blotting
Immobilon®Forte Western HRP Substrate WBLUF0500 500 mL
Immobilon®Crescendo Western HRP Substrate WBLUR0500 500 mL
Immobilon®Classico Western HRP Substrate WBLUC0500 500 mL
Immobilon®Western HRP Substrate WBKLS0500 500 mL
Immobilon®ECL Ultra Western HRP Substrate WBULS0500 500 mL
TMB, insoluble 613548 100 mL
Immobilon®Signal Enhancer for Immunodetection WBSH0500 500 mL
Immunoblot Blocking Reagent 20-200 20 g
Re-Blot™ Plus Strong Antibody Stripping Solution, 10X 2504 50 mL
Immobilon® Block-CH Buffer WBAVDCH01 500 mL
Immobilon® Block-FL Buffer WBAVDFL01 500 mL
Immobilon® Block-PO Buffer WBAVDP001 500 mL
BLOT-QuickBlocker™ Reagent WB57-175GM 175 grams
Probumin®Bovine Serum Albumin 820473 100 g
5% Alkali-soluble Casein 70955 225 mL
Components For Immunohistochemistry (IHC) Procedures
SNAP i.d.®2.0 Immunohistochemistry Frame SNAP2FRIHC 1
SNAP i.d.®2.0 IHC Slide Holder SNAP2SH 24
Accessories
Filter forceps, blunt end, stainless steel XX6200006P 3
Vacuum filtering flask, 1 L XX1004705 1
SNAP i.d.®2.0 Blot Roller SNAP2RL 1
High Output Pump, 115 Volts, 60 Hz WP6211560 1
High Output Pump, 100 Volts, 50/60 Hz WP6210060 1
High Output Pump, 220 Volts, 50 Hz WP6222050 1
Vacuum tubing, 6.4 mm ID × 3 m (1⁄4in. ID × 10 ft) MSVMHTS09 1
Millex®-FA filter unit, 1.0 µm, hydrophobic PTFE, 50 mm SLFA05010 10
Antibodies
Primary and secondary antibodies go to www.sigmaaldrich.com/antibodies
Table of contents
Other Millipore Sigma Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands

Universal Medical
Universal Medical Lab Improvements Cap Track user manual
SCHOTT
SCHOTT TITRONIC universal operating instructions

Thermo Scientific
Thermo Scientific TSQ Quantum GC user guide

Grant
Grant Optima T100 operating manual
DLab
DLab Liquid Handling HiPette user manual

Bruker
Bruker DektakXT user manual