-10-
■ Cleaning Instructions for the Endoscope Holder
Caution: Wear protective gloves, clothing, and a face mask for cleaning
of contaminated endoscope holder.
Caution: Hot water (greater than 49 degrees C or 120 degrees F) will
alter organic materials (blood and tissue), making them
difficult to remove.
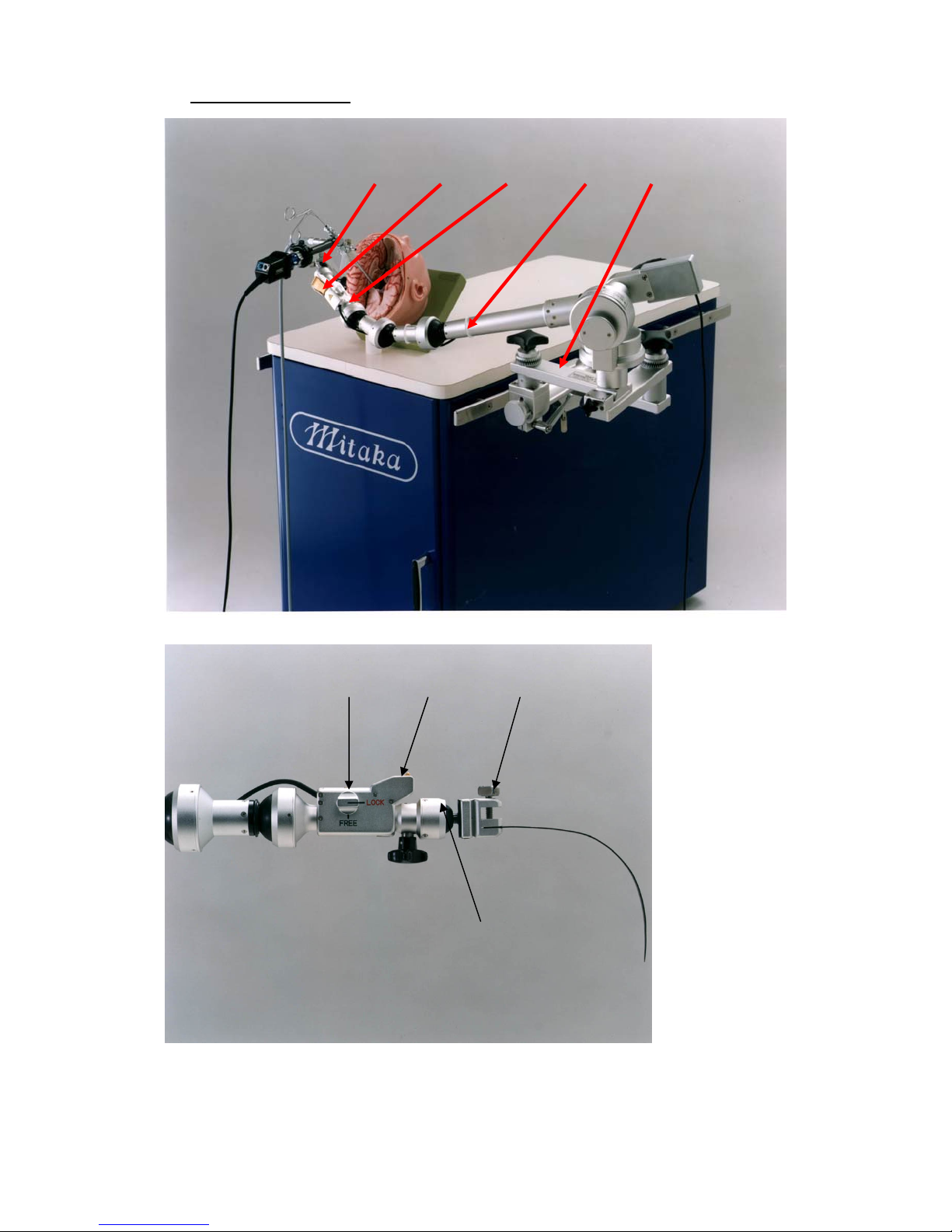
1. The endoscope holder should be disassembled.
2. Thoroughly rinse all parts to remove all gross debris.
3. Manual cleaning is recommended. However, difficult to reach areas can be
cleaned using an ultrasonic cleaner. It is important to follow the manufacturer’s
instructions for operating the ultrasonic cleaner.
4. Completely immerse the endoscope holder in a neutral pH enzymatic cleaning
solution (e.g., Enzol, Metrizyme or equivalent per manufacturer’s instructions)
and distilled water. Use water that is less than 49 degrees C or 120 degrees F to
clean the endoscope holder; blood and tissue will become difficult to remove
using hot water. Mitaka does not recommend the use of detergents alone, as they
contain high concentrations of surfactants which can leave a film on the
endoscope holder.
5. Remove any residual blood, protein material and contaminants from the
endoscope holder with brushes, sponges, soft cloths or a cotton cloth applicator.
6. Clean the inside of the sheaths and outer tubes with the appropriate size cleaning
brushes.
7. Cleaning brushes should be cleaned and subjected to high level disinfectant or
sterilization daily.
8. Triple rinse the endoscope holder with distilled water for a minimum of one
minute for each rinse. The rinse water should be discarded at the end of each
rinse, as it will be contaminated with the cleaning solution. Thorough rinsing of
the endoscope holder is necessary for removing any debris or detergent which
could interfere with sterilization.
9. Dry the endoscope holder with a lint-free soft cloth or filtered compressed air.