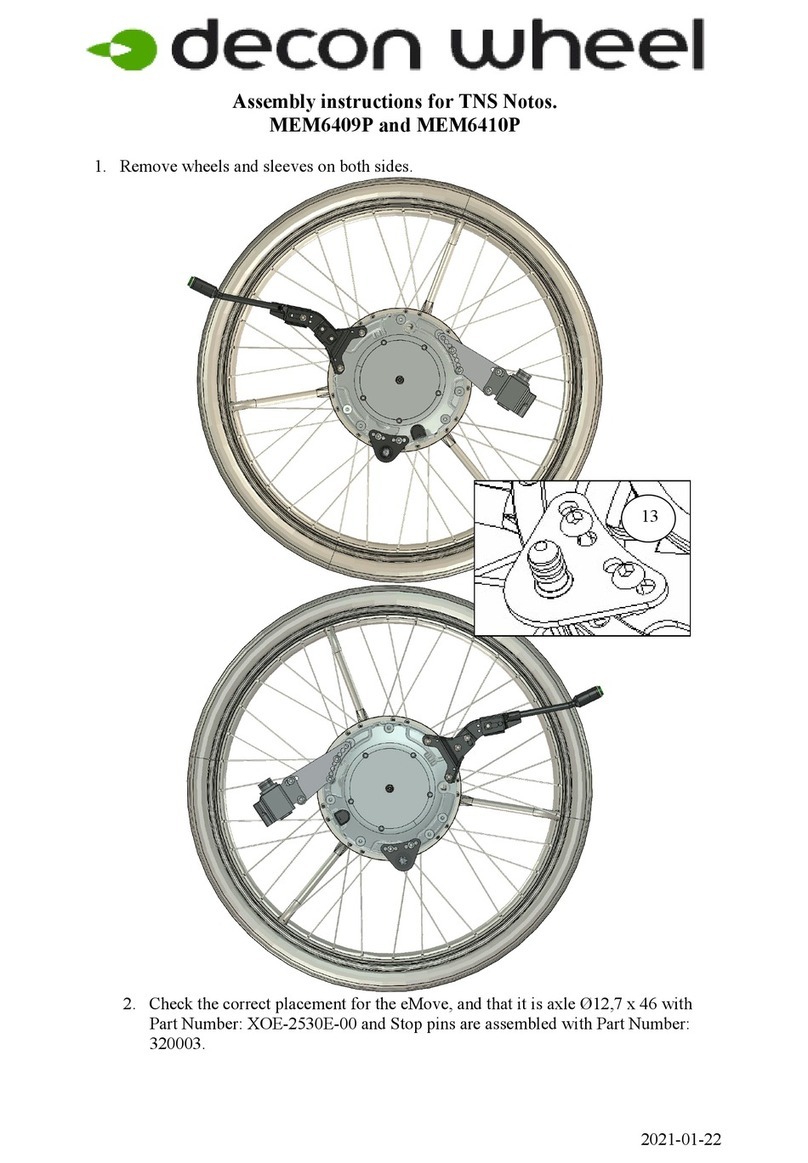
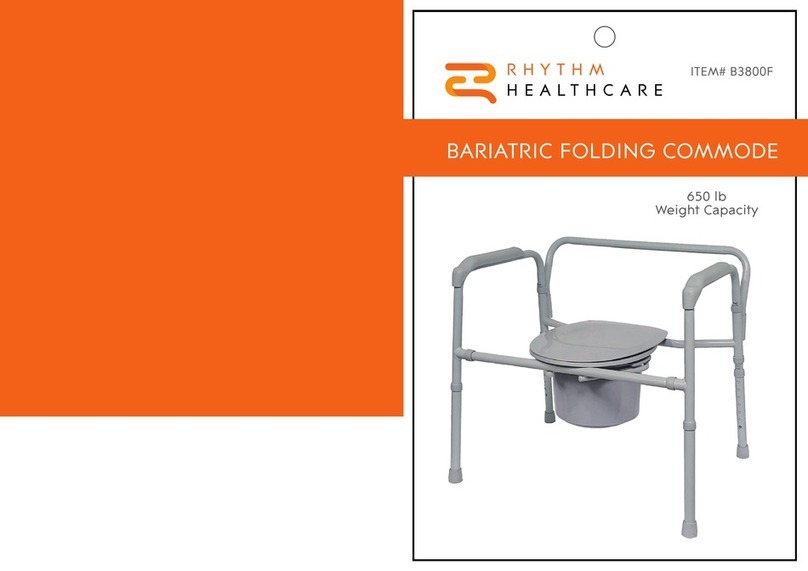
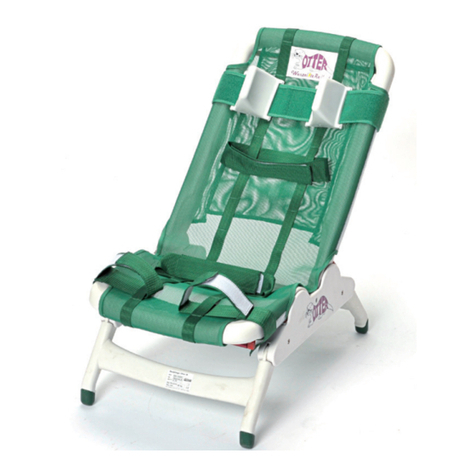
2
By reading this document beyond this section, and/or using the therapeutic medical device (“CrawlAhead”) herein (hereafter “Device”), to the
fullest extent permitted by law, the parent, guardian, clinician, and/or Therapist responsible for any child or patient under their care who is
allowed to be in proximity of the said Device (hereafter “Patient”) shall indemnify, defend and hold harmless Mobility Research Inc., their legal
representatives, clients, associates, assigns, sub-contractors, corporate bodies, agents, and employees (hereafter collectively “Mobility
Research”), from and against all claims arising out of or resulting from the use of said device, with and on the named Patient. The term “Claim” as
used in this agreement is defined as any financial loss, claim, suit, action, damage, or expense, including but not limited to attorney’s fees,
attributable for bodily injury, sickness, disease or death, or injury to or destruction of tangible property including loss of use resulting there from.
Therapist’s adult user’s obligation to indemnify, defend, and hold harmless includes any claim by Therapist’s and adult’s legal representatives,
clients, associates, assigns, agents, employees, or representatives.
Therapist, parent, or any other adult user of the “device”, agrees that they are fully aware of risks and hazards connected with the use of said
”device”, and elect voluntarily to have the Patient participate in such use, knowing that it may be hazardous to Patient. Therapist and adult user
understands that the potential risks and hazards include failure of the Device being used due to inadequate/unsafe design, improper use, and/or
any number of possible sources including those known, unknown or anticipated by Mobility Research, regardless whether or not explicitly
disclosed to any user by Mobility Research. Therapist/adult user agree to voluntarily assume full and complete responsibility for any risks of loss,
property damage or injury, including death, that may be sustained by Patient, or any loss or damage to property owned by said adult users, as a
result of being engaged in using the device, whether caused by the negligence of Mobility Research or otherwise. Such adult users understand
and agree that they will not be compensated in any manner whatsoever for Patient’s participation in said Testing.
The statements in this document have not been approved by the Food & Drug Administration (FDA). This product has not been approved by the
Food & Drug Administration to prevent, cure, treat, or diagnose any disease.
Absolutely none of the statements in this document should be construed as medical advice. The information provided in this document is for
informational purposes only and is not intended as a substitute for advice from a physician or other health care professional.
The information in this document and/or the Device is describes should not be used for diagnosis or treatment of any health problem or for
prescription of any sort of medical treatment. You should consult with a licensed healthcare professional before considering beginning any type
of therapy, or if you have, or suspect the patient with whom you intend to use it may have a health problem. Those seeking treatment for a
specific disease should consult a qualified physician.
Mobility Research reserves the right to make changes without notice in design, specifications, and models. Mobility Research provides no
warranties or guarantees for this Device.
The information in this manual is confidential and may not be disclosed to third parties without the prior written consent of Mobility Research.
This user’s guide and therapeutic device as described within are provided as-is, without warranty as to their performance, merchantability, or
fitness for any particular purpose. The entire risk as to the safety, results, and performance of this product is assumed by the parent, guardian, or
clinician specifically by way of example but not limitation, in the event that the Therapist or the Patient have history of medical conditions,
muscular disorders, infirmities or are not in good health, Therapist should consult with the Patient’s physician, clinician, and/or Therapist before
using this Device.
Mobility Research shall not be liable for indirect, social, or consequential damages resulting of the use of this Device.
This user’s manual is provided as a clinical aid to assist properly licensed medical professionals in the usage of this device. As part of this
professional usage, the medical professional must use their professional judgment in making any final determinations in product usage and
technique. In doing so, the medical professional should rely on their own training and experience and should conduct a thorough review of
pertinent medical literature and the product’s Directions For Use or User’s Manual.
Therapist/adult user agrees that this release is irrevocable, worldwide and perpetual, and will be governed by the laws (excluding the law of
conflicts) of Arizona. Therapist and any adult user hereby warrants that he/she is a legal competent adult and a parent, legally appointed guardian
of the Patient, and/or the Patient’s clinician/Therapist, and that they have every right to contract for the Patient in the above regard. Therapist/
adult user states further that he/she has read the above authorization, release, and agreement, prior to use, and that they are fully familiar with
its contents. This release shall be binding upon the Patient and Therapist, adult user and their respective heirs, legal representatives, associates,
and assigns.
US Patent# 9,445,968 B1
! LEGAL DISCLAIMER