Moveo! ExoBand User manual

ExoBand


USE AND MAINTENANCE MANUAL
Hip orthosis
ExoBand
Version: 2.2 - Date of latest version of the manual May 2022
Table of Contents
DECLARATION OF CONFORMITY ................................................................................................................... 2
MANUFACTURER'S INFORMATION ............................................................................................................... 2
AUTHORIZED TECHNICAL SERVICE ................................................................................................................ 2
THE MANUAL................................................................................................................................................ 2
Conventions .................................................................................................................................................. 3
SYMBOLS ...................................................................................................................................................... 4
WARRANTY .................................................................................................................................................. 4
GENERAL SAFETY RULES ............................................................................................................................... 5
Warning ........................................................................................................................................................ 5
DESCRIPTION OF THE MEDICAL DEVICE ........................................................................................................ 5
TECHNICAL DATA .......................................................................................................................................... 6
INTENDED USE AND UNINTENDED USE OF THE DEVICE ............................................................................ 7
Sphere of application and intended use ......................................................................................................... 7
Unintended use............................................................................................................................................. 7
Limitations of the medical device ................................................................................................................. 7
RESIDUAL RISKS ............................................................................................................................................ 7
Adverse effects ............................................................................................................................................. 8
Contraindications .......................................................................................................................................... 8
HANDLING .................................................................................................................................................... 8
USE ............................................................................................................................................................... 9
Storage ......................................................................................................................................................... 9
Application.................................................................................................................................................... 9
Precautions ................................................................................................................................................. 11
MAINTENANCE ........................................................................................................................................... 14
Safety ......................................................................................................................................................... 14
Periodic maintenance ................................................................................................................................. 14
Washing ...................................................................................................................................................... 16
Special maintenance ................................................................................................................................... 16
Disposal ...................................................................................................................................................... 16
MOVEO S.R.L.
Use and Maintenance
page 2
DECLARATION OF CONFORMITY
The medical device described in this documentation is sold with the declaration of conformity in compliance with the
regulations in force in Europe.
NOTE
BEFORE USING THE DEVICE, MAKE SURE THAT THE DECLARATION OF CONFORMITY HAS
BEEN DELIVERED TOGETHER WITH IT.
MANUFACTURER'S INFORMATION
Company name
Moveo S.r.l.
Registered office:
Via Monsignor Fortin 37/38, 35128 Padua - Italy
VAT no.
05236760285
Tel. Office
+39 049 261 44 27
Mobile
+39 3914590627
E-mail
info@moveowalks.com
Website
www.moveowalks.com
AUTHORISED TECHNICAL SERVICE
Technical operations on the hip orthosis may be carried out exclusively by authorized and qualified
personnel of Moveo S.r.l. or its representative.
THE MANUAL
NOTE
READ THIS MANUAL CAREFULLY BEFORE CARRYING OUT ANY OPERATION WITH THE
DEVICE.
This manual contains instructions for the use and maintenance of the ExoBand Hip Orthosis. The manual is composed of
sections, each of which addresses different topics, subdivided into chapters and paragraphs.
The table of contents lists all the topics covered in this manual. Each page is numbered progressively. This manual
contains all the instruction for the proper use, maintenance, and storage of the hip orthosis during its lifespan.
This manual contains confidential information and must not be provided to third parties, even partially, for any use and
in any form, without the prior written consent of the manufacturer.
Moveo S.r.l. declares that the information contained in this manual is consistent with the technical and safety
specifications of the medical device to which the manual refers. A certified copy of this manual is contained in the device's
technical file kept by Moveo S.r.l.
Use and Maintenance
page 3
Moveo S.r.l. does not recognize any documentation that has not been produced, released, or distributed by Moveo S.r.l.
itself or by one of its authorized representatives.
This manual, like the entire technical file, will be kept by the manufacturer for the period provided for by law.
During this period, customers may request a copy of the documentation provided with the product at the time of
purchase.
The entire technical file will be available to supervisory authorities, which may request a copy thereof, for the period
provided for by law.
After this period, whoever uses and/or handles the product must make sure that both the product and the
documentation comply with the laws in force.
Conventions
In order to facilitate comprehension of the topics covered, the manual utilizes graphic and typographic symbols and
conventions as described below.
Graphic warning symbols
WARNING
WARNINGS INDICATE PROCEDURES WHICH MUST BE STRICTLY FOLLOWED, AS TOTAL OR
PARTIAL NON-COMPLIANCE MAY CAUSE DAMAGE TO THE MEDICAL DEVICE AND MAY
EXPOSE THE PATIENT TO DANGER.
DANGER
INDICATIONS OF DANGER REGARD PROCEDURES WHICH MUST BE FOLLOWED WITHOUT
EXCEPTIONS, AS TOTAL OR PARTIAL NON-COMPLIANCE MAY CAUSE DAMAGE OR INJURY
TO THE PATIENT OR OTHERS NEARBY.
NOTE
NOTES CONTAIN CRITICAL INFORMATION HIGHLIGHTED APART FROM THE TEXT TO
WHICH THEY REFER

Use and Maintenance
page 4
SYMBOLS
Lot number
Date of manufacture
Manufacturer
Agent
Read the manual before use
CE mark
WARRANTY
The terms of the warranty, listed in full in the purchase contract, are only valid if the medical device is used as intended
and exclusively for the purposes for which it was designed.
Except for the routine and special maintenance operations described in the MAINTENANCE section and carried out in
accordance with the indicated procedures, any repair or modification made to the device by the user or by unauthorized
companies will void the warranty.
The warranty does not extend to damage caused by incompetence or negligence in the use of the device, or as a result
of poor or lacking maintenance.
The products we sell are covered by a warranty under the following conditions:
1
The warranty is valid for a period of twelve (12) months for juridical persons and twenty-four (24) months for natural persons,
starting from the date indicated
on the invoice. The warranty
on
parts
subject to wear (mechanical
components) is
valid for a
period
of six (6) months from the date indicated on the invoice.
2
The manufacturer undertakes to replace all defective parts at its own discretion, only after inspecting the device/the parts in
question.
3
Shipping costs are always borne by the purchaser if the terms of the warranty are not met.
4
During the warranty period, the replaced components become the property of the manufacturer.
5
Only the original purchaser who has complied with the maintenance instructions contained in this manual can benefit from this
warranty. Our warranty liability expires when the original purchaser relinquishes ownership of the device, or if the device is modified.
6
The warranty does not cover damage resulting from excessive stress (use of the device after a fault or defect has been found, use of
unsuitable operating methods, as well as failure to observe the use and maintenance instructions).
7
The manufacturer is not liable for difficulties arising in the resale or use of the device abroad as a result of the provisions of the laws
in force in the country where the device was sold.
Use and Maintenance
page 5
Note: If it is deemed necessary to use the warranty, please indicate the following information:
1
Type
2
Date of purchase (submit the purchase receipt)
3
Detailed description of the problem
GENERAL SAFETY RULES
Warning
This section describes the general safety rules to be observed during any operation performed with the medical device.
The operating procedures described in the following sections must be carried out in accordance with the operating
methods indicated and the general safety provisions provided in this section.
NOTE
THE MANUFACTURER SHALL NOT BE LIABLE FOR ACCIDENTS OR DAMAGE CAUSED BY
INAPPROPRIATE USE OF THE PRODUCT, OR BY EVEN PARTIAL FAILURE TO COMPLY WITH
THE SAFETY RULES AND OPERATING PROCEDURES DESCRIBED IN THIS MANUAL.
Failure to comply with the instructions for the use and maintenance of the medical device contained in this manual also
voids the warranty.
DESCRIPTION OF THE MEDICAL DEVICE
WARNINGS
The medical device must be prescribed and used under medical supervision. The orthopedic specialist is the expert of
reference both for the application and for information relating to safe use. To guarantee the efficacy, tolerability and
proper operation of the product, it must be applied and adjusted with the utmost accuracy. Any modification of the
structure of the device or adjustment of the latter must be prescribed by a doctor and implemented by an orthopedic
specialist. In hypersensitive individuals, direct contact with the skin may cause redness or irritation. If you experience
pain, swelling, or any other abnormal reaction, contact your doctor immediately.

Use and Maintenance
page 6
The medical device is designed exclusively to support human walking. Any unintended use is not recommended (such as
using it when travelling in a car, riding a motorbike or bicycle, or even in the water, etc.). Any damage resulting from
improper use is the responsibility of the customer.
The device consists of a belt, two leg loops and two tensioners which together with a toothed guide join all these
elements. The device is available in 5 sizes (XS, S, M, L, and XL).
Class I Medical device.
TECHNICAL DATA
Belt and mechanism:
•
Airshell 100% Polyester
•
AL
•
FE 10B21
•
Cordura® 40% Cotton Lycra and 60% Polyamide
•
Nylon 618 fiber
•
Polyvinyl Chloride (PVC) 82% - Polyethersulfone (PES) 18%
•
Spandex 10% - Polyester 90%
•
Brass with nickel barrel plating finish
•
Polyester 70% - Spandex 30%
•
Polyoxymethylene (POM)
•
100% polypropylene
•
Polyamide/polyurethane resin
•
87% Polyamide - 13% acrylic
•
Nylon 37% - Polyester 33% - Rubber and Silicone 30%
•
NY6 Buckles (Polimid B AV Natural HF)
•
Adhesive interlining 100% Polyamide
•
Tough Resin FLTOTL05 / PA12
•
Tough RPU 70
Leg loops:
•
Airshell 100% Polyester
•
Cordura® 40% Cotton Lycra and 60% Polyamide
•
Acrylic (LOCTITE 243)
•
Galvanized steel
•
Aluminum alloy sleeves
•
Polyamide/polyurethane resin
•
87% Polyamide - 13% acrylic
•
Nylon 37% - Polyester 33% - Rubber and Silicone 30%
•
Aluminum (Nickel) and plastic
•
Polyester 75% - Rubber 25%
•
Polyvinyl Chloride (PVC) 82% - Polyethersulfone (PES) 18%
•
Stainless steel
•
Adhesive interlining 100% Polyamide
•
Polyester 70% - Spandex 30%
•
Tough Resin FLTOTL05 / PA12
•
Tough RPU 70
•
Polytetrafluoroethylene (PTFE)
•
Dyneema® SK78 - HT Polyester
Features: Belts designed to adapt the product to different human morphologies.

Use and Maintenance
page 7
INTENDED USE AND UNINTENDED USE OF THE DEVICE
Sphere of application and intended use
The medical device must be used exclusively as:
•
a walking aid for the elderly and people with motor impairments caused by disabling diseases and/or health
conditions
•
an aid to improve improper or pathological posture.
NOTE
THE MANUFACTURER IS NOT LIABLE FOR ACCIDENTS OR DAMAGE CONSEQUENTIAL TO
THE UNINTENDED USE OF THE PRODUCT. ANY UNINTENDED USE OF THE DEVICE WILL
ALSO VOID THE WARRANTY.
Unintended use
The medical device is not designed for any use other than those described in the paragraph "SPHERE OF APPLICATION
AND INTENDED USE". Furthermore, the following uses are absolutely forbidden:
• use of the medical device by individuals who have not read the manual
• use by children
• use by pregnant women
• use in direct contact with the skin.
A medical prescription is recommended for use by individuals suffering from serious diseases such as musculoskeletal
system tumors, neuromuscular pathologies, spinal pathologies, hernias, etc.
Limitations of the medical device
• The medical device is not able to cure serious spinal deformations (dysmorphisms) or other dysmorphisms (it
can only reduce the outcomes).
• The medical device can be used for long periods of time, that is, until it shows signs of wear or damage that are
such as to affect its structure, safety, and operation. In this case and when in doubt, consult the seller immediately.
• The medical device can reach its maximum efficiency after a gradual period of use according to the
psychophysical conditions of the user.
RESIDUAL RISKS
During the design phase Moveo S.r.l. conducted an in-depth risk analysis of the device. This analysis demonstrated that
some risks cannot be eliminated due to their nature. These residual risks are addressed in this manual and indications
regarding how to prevent them are provided throughout the same. It is therefore important that anyone who uses or
handles the device has read and understood the manual beforehand.
NOTE
THE MANUFACTURER IS NOT LIABLE FOR ACCIDENTS OR DAMAGE CONSEQUENTIAL TO
ANY UNINTENDED OR NEGLIGENT USE OF THE PRODUCT.

Use and Maintenance
page 8
In particular:
•
Do not make any modification to the medical device. Any damage resulting from the use of the device if this has
been improperly modified by an unauthorized person releases the manufacturer from
•
any liability.
•
Keep this manual in good condition and easily available as this is necessary for the proper and safe use of the
hip orthosis.
•
If the structure of the device has sharp edges following an accidental collision or fall or a part worn enough to
make it hazardous, contact an authorized assistance service and follow the instructions provided. +++
Adverse effects
In the analysis and experimentation phase, no adverse effects associated with the use of the medical device were
detected. Sores, redness, numbness or tingling can occur in case of improper use of the medical device.
Contraindications
•
The length of time the medical device is used must be proportional to the psychophysical conditions of the user.
•
The medical device may cause muscle pain and/or fatigue when used without observing the indications
contained in "Limitations of the medical device."
•
Elastic bands, as such, if stretched and then released can cause pain or injury. They must therefore be carefully
applied and handled to prevent their uncontrolled release (as with any elastic material).
•
Keep to and ensure compliance with the instructions provided above in section "Unintended use of the medical
device".
HANDLING
Carefully check the integrity of the device and its parts upon receipt. If there are any signs of damage, deformation,
impact, or missing parts, inform the manufacturer before proceeding with any subsequent operation. The medical device
must always be handled with care to avoid damaging it and making it unusable and/or dangerous. It can be handled
manually without any problem.
Procedure for reporting damage or defects
If any damage or defects are found, stop the assembly process and report the nature of the damage or defect found to
Moveo S.r.l.’s customer support team.

Use and Maintenance
page 9
USE
Storage
•
Store the hip orthosis indoors, away from the elements and humidity.
•
Store the hip orthosis away from heat sources, open flames, and direct sunlight.
•
Store the leg loop tensioners properly.
•
Store the product in a box and/or drawer.
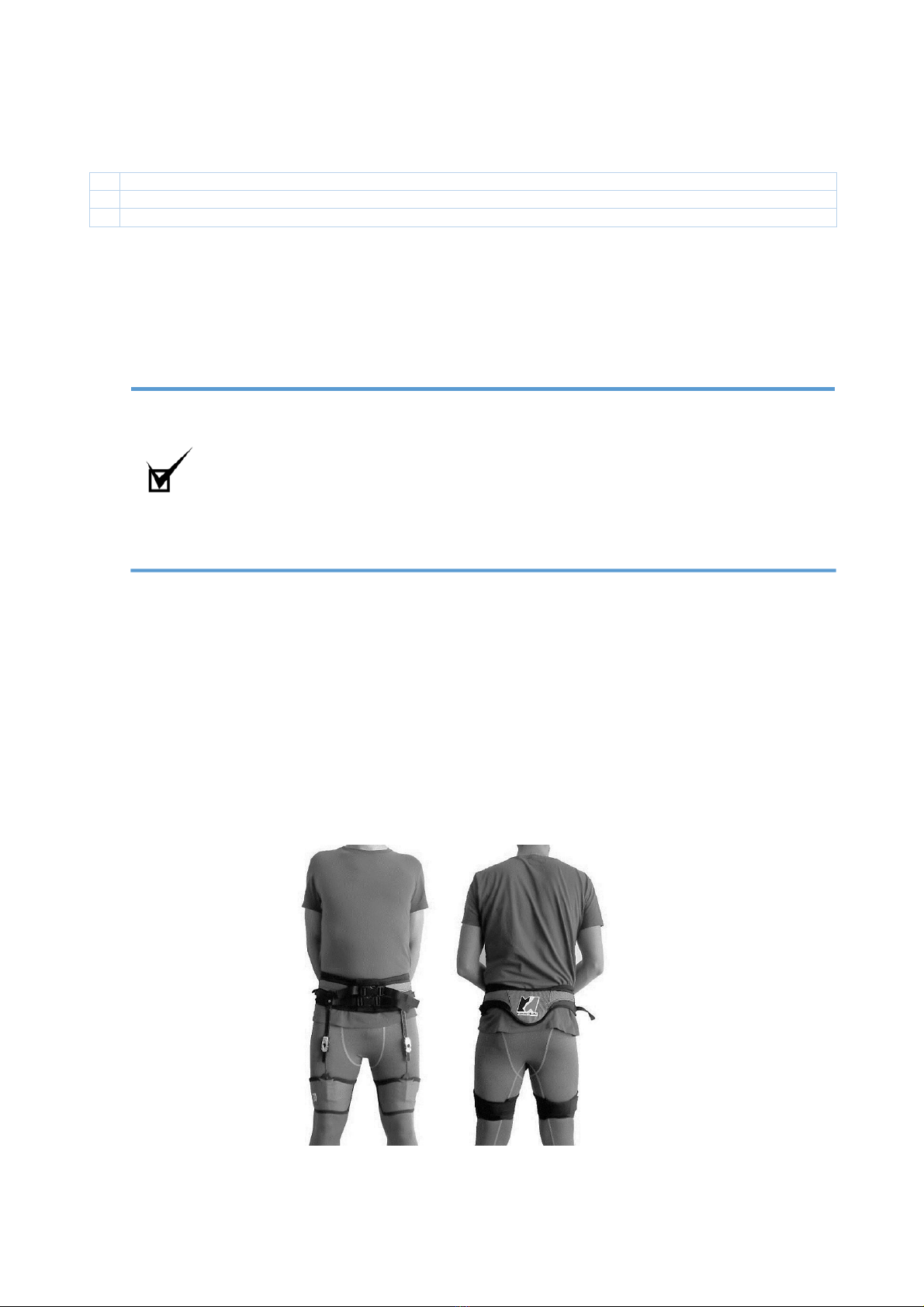
Use
1 Position the belt at the height of the anterior iliac crests (ASIS)
2 Carefully tighten the Velcro strap of the belt keeping the sliding buckles (A) at the height of the ASIS
3 Close the side release buckles (B) and tighten the fabric straps (C), tightening the belt and anchoring it to the pelvis
without creating excessive compression
4 Once the belt is tightened, check that the sliding buckles (A) are aligned over the ASIS
5 Align the rear end of the belt with the spinal column
6-7 Once the sliding buckles (A) are in place, fasten the strip with the pressure buckle (D) located on the side of the
belt
8 Hook the tensioner (E) of the leg loops to the toothed guide (F) of the belt. Place the leg loops close to the kneecap.
9 Fasten the leg loops starting from the two inner fabric wings (G) and tighten them carefully. Fasten the leg loops on
the soft part of the Velcro strap (H).
10 Pull and attach the elastic covering strip (K) to the soft part of the Velcro strap (H).
11-12 Adjust the tension of the mechanism (L) by lifting the metal tab of the tensioner (E) by 90 degrees until you hear it
"click."

Use and Maintenance
page 10

Use and Maintenance
page 11
Warning:
●
The medical device must be worn over clothing.
●
Trousers with raised rivets and/or buttons can damage the belt.
●
Do not overtighten the mechanism (L) as this may make the device uncomfortable.
●
To loosen or release the mechanism (L), press the button in the center of the tensioner (E) and push it
downwards as shown in the figures below.
Precautions
Improper use may damage the device.
Front and rear belt positioning
The belt must be positioned at the correct height as illustrated in section "USE" of this manual. The proper and improper
positioning of the belt is shown below.

Use and Maintenance
page 12
Excessive tension of the mechanism
Excessive tension of the mechanism results in the irregular alignment of the belt as shown in the figures below. If the
device is in this configuration, decrease the tension of the mechanism.
Inserting the tensioner into the toothed guide
Insert the tensioner into the toothed guide by proceeding as shown in the figures, holding it on its sides.
Do not squeeze the spring tensioner as, once inserted, it could prevent the tensioner from sliding and damage the sliding
mechanism.
Use and Maintenance
page 13
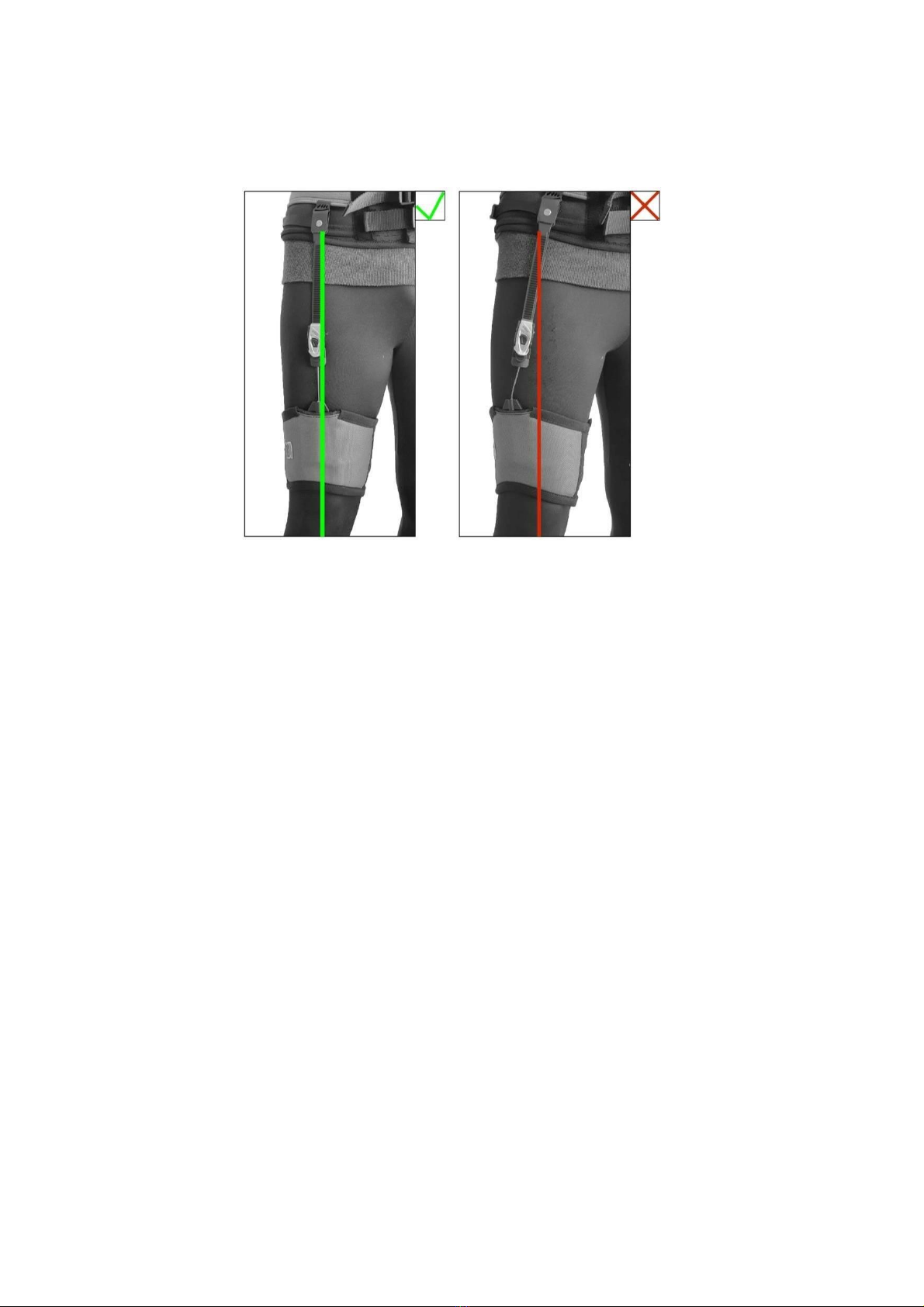
Aligning the leg loops
Always make sure that the leg loops are properly aligned with the belt as shown in the figure. Misalignment may cause
the cable to break and consequently make the device unusable.

Use and Maintenance
page 14
MAINTENANCE
Safety
It is absolutely necessary to read the manual before carrying out any maintenance operation.
Periodic maintenance
The hip orthosis can be periodically cleaned to eliminate dirt and bad smells. To wash it, remove the mechanism (L)
following the procedures below:
1-
Remove the screw cap (M) located on the upper part of the leg loop (Figure 1 - 1A).
2-
Gently turn the screw cap (M) counterclockwise as shown in Figure 2 until reaching position 2A.
3-
Remove the elastic element and store it in a safe place, taking care not to damage the cable (Figure 3).
4- Situation after removing the elastic element.

Use and Maintenance
page 15
5-
After washing the device, screw the screw cap (M) back into its seat, making sure to insert it properly. Insert the screw
cap (M) in the direction shown in Figure 5A.
6-
Rotate it clockwise until reaching the position shown in Figure 6A.
7- Final result.

Use and Maintenance
page 16
Washing
Machine wash in cold water on a delicate cycle after removing the elastic components from the leg loops as described
above. Leave to dry on a drying rack, away from heat sources. Do not use dryer. Do not use fabric softener. Do not dry
clean. Do not iron.
Special maintenance
Special maintenance is required in case of failure or breakage following unforeseeable accidents or inappropriate use of
the device.
The situations that might occur are entirely unpredictable; therefore, it is not possible to describe appropriate
countermeasures.
If necessary, consult with the Moveo S.r.l. technical service for appropriate instructions for the specific situation.
All special maintenance activities must in any case be carried out by specialized and authorized personnel, under
penalty of voiding the warranty.
Disposal
Any reuse of parts of the medical device is the responsibility of the user. The materials with which the medical device is
made do not require special disposal procedures. Refer to local regulations for the disposal of unsorted residual waste.
Remove the plastic and metal parts from the medical device and dispose of them as prescribed by law.
Do not dispose of or abandon the product in the environment for any reason whatsoever.
NOTE
THE MANUFACTURER IS NOT LIABLE FOR DAMAGE CAUSED BY THE DEVICE IF THIS IS NOT
USED IN ITS COMPLETE CONFIGURATION AND FOR ITS INTENDED PURPOSE AS SPECIFIED
IN THIS MANUAL.
THE MANUFACTURER IS NOT LIABLE FOR ANY PERSONAL INJURY OR DAMAGE TO
PROPERTY RESULTING FROM THE RECOVERY AND REUSE OF PARTS ONCE THE DEVICE HAS
BEEN DISMANTLED.

Declaration of conformity
The undersigned Mr. Fausto Antonio Panizzolo
in his capacity as the legal representative of Moveo S.r.l.
with registered office in: Via Monsignor Fortin 37/38, 35128 Padua, Italy
VAT number: 05236760285
declares
that the product: hip orthosis
Model and code: ExoBand
Medical device class: I
Date of manufacture: January 2022
Lot No:
was manufactured in compliance with the following directives and standards:
•
Regulation (EU) no. 745/2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) no. 178/2002 and
Regulation (EC) no. 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC
•
IEC 61882:2016 Standard: Hazard and operability studies (HAZOP studies) - Application guide
•
EN 980:2009 Symbols for use in the labelling of medical devices
•
EN ISO 15223-1: 2012 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied
- Part 1: General requirements
•
EN 1041:2009- Information supplied by the manufacturer of medical devices
•
EN 14971:2012 - Application of risk management to medical devices
•
Directive 93/42/EEC, Leg. Decree 24/02/97 no. 46, Leg. Decree 25/02/98 no. 95 Council Directive on medical devices -
Implementation of Directive 93/42/EEC on medical devices. Changes to Leg. Decree 24 February 1997 no. 46
(which will coexist
with the new Regulation until 2020)
•
Directive 2007/47/EEC Leg. Decree 25/01/10 no. 37 Council Directive on medical devices - Implementation of Directive
93/42/EEC on medical devices. (which will coexist with the new Regulation until 2020)
•
Directive 2001/95/EC also known as the general product safety directive
•
Regulation (EU) No 1007/2011 of the European Parliament and of the Council of 27 September 2011 on textile fibre names and
related labelling and marking of the fiber composition of textile products.
It therefore complies with current directives and standards.
This declaration of conformity is issued under the sole responsibility of the manufacturer.
Place: Padova
Date: 23 December 2019
Rev. 1
Signature:
Via Lauro, 95 Cadoneghe Padova Italy
Consulenti e Periti
per:
Guardia di Finanza, Tributaria, Autorità Doganali, Carabinieri,
Polizia di Stato, Unioncamere, Courts of law.
The proper composition of the technical file as well as the documents prepared by C&C s.a.s. have been verified by
Renato Carraro, engineer.
Basic UDI-DI: 805934045EXOBAND0003N
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











