applicator seal
obturator support
obturator
applicator
location of IMPLANON
cannula
IMPLANON
4 cm
needle
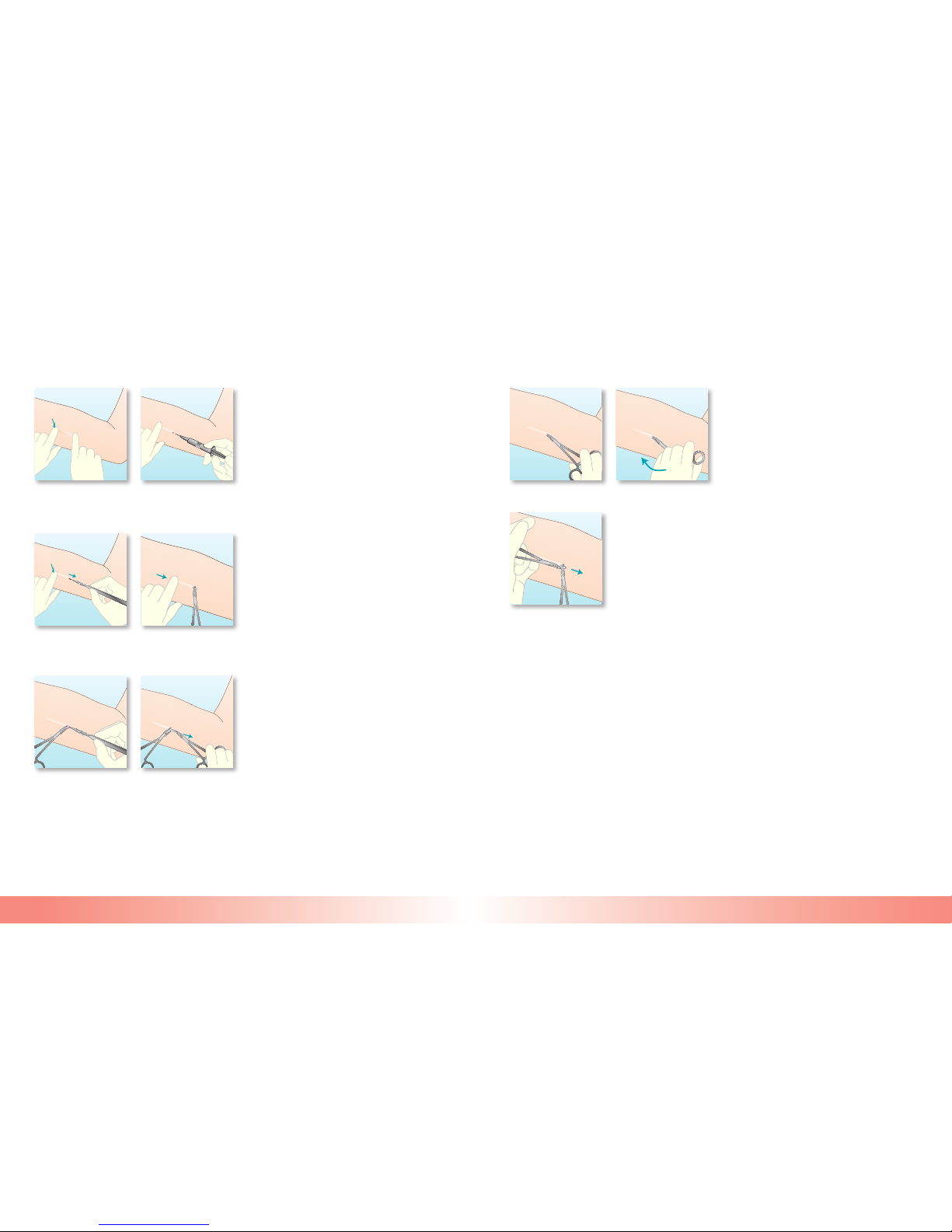
How to Insert IMPLANON
•Inser tionofIMPLANONshouldbeperformedunder
asepticconditions,andonlybyahealthcareprovider
whoisfamiliarwiththeprocedure.
•Inser tionofIMPLANONisperformedwiththespecially
designedapplicator.Theuseofthisapplicatordiffers
substantiallyfromthatofaclassicalsyringe.Adrawing
ofadismantledapplicatoranditsindividualcomponents
(eg,cannula,obturator,andneedlewithdouble-angled
bevel)isshowninthisleaettoclarifytheirspecicfunctions.
•TheprocedureusedforinsertionofIMPLANON
isoppositetogivinganinjection.Wheninserting
IMPLANON,theobturatormustremainxedwhilethe
cannula(needle)isretractedfromthearm.
•Allowthesubjecttolieonherbackwithhernon-dominant
arm(thearmwhichthewomandoesnotuseforwriting)
turnedoutwardandbentattheelbow.
•Tominimizetheriskofneuralorvasculardamage,
IMPLANONshouldbeinsertedattheinnersideofthe
non-dominantupperarmabout8to10cmabovethe
medialepicondyleofthehumerus.
•IMPLANONshouldbeinsertedsubdermally,
ie,justundertheskin(subcutaneously).
•WhenIMPLANONisinsertedtoodeeply
(intramuscularlyorinthefascia),thismaycause
neuralorvasculardamage.Toodeepinsertions
havebeenassociatedwithparesthesia(dueto
neuraldamage)andmigrationoftheimplant(due
tointramuscularorfascialinsertion),andinrare
caseswithintravascularinsertion.Moreover,when
theimplantisinsertedtoodeeply,itmaynotbe
palpableandlocalizationand /orremovalcanbe
difcultlater.
•Marktheinsertionsite.
•Cleantheinsertionsitewithanantiseptic.
•Anesthetizewithananestheticspray,orwith2mLoflidocaine
(1%)appliedjustundertheskinalongthe“insertioncanal.”
•Removethesteriledisposableapplicatorcarrying
IMPLANONfromitsblister.
•Whilekeepingtheshieldontheneedle,visuallyverifythe
presenceoftheimplant,seenasawhitebodyinsidethe
needletip.Iftheimplantisnotseen,tapthetopofthe
needleshieldagainstarmsurfacetobringtheimplantinto
theneedletip.Followingvisualconrmation,theimplant
shouldbeloweredbackintotheneedlebytappingitback
intotheneedletip.Theneedleshieldcannowberemoved.
•Pleasenotethattheimplantcanfalloutoftheneedleprior
toinsertion.Therefore,alwaysholdtheapplicatorinthe
upwardposition(ie,withtheneedlepointedupward)until
thetimeofinsertion.Thisistopreventtheimplantfrom
droppingout.Keeptheneedleandtheimplantsterile.
Ifcontaminationoccurs,anewpackagewithanewsterile
applicatormustbeused.
ApplicatorforIMPLANON
IMPLANON™:ClinicalInformation
•IMPLANONisasubdermal,long-acting,progestagen-only
contraceptiveeffectiveforupto3years.
•ThemechanismsofactionofIMPLANONincludeinhibition
ofovulationandincreasesintheviscosityofcervicalmucus.
•Efcacydoesnotdependondaily,weekly,ormonthly
self-administration.
– IMPLANONiseffectivefromdayone,wheninser ted
accordingtoinstructionsinthelabel.
•Nomethodofcontraceptionis100%effective—
IMPLANONisover99%effectivewheninserted
correctly. 1
– Studiesshowacontinuationrateof82%after1yearofuse.2
– 11%ofwomenstudieddiscontinuedIMPLANONdue
tobleedingirregularities.
•Bleedingirregularitiesmayincludeamenorrheaorinfrequent,
frequent,and/orprolongedbleeding.Information,counseling,
andtheuseofableedingdiarycanimprovethewoman’s
acceptanceofableedingpattern.
•FollowingremovalofIMPLANON,thehormoneisbelow
detectablelevelswithin7days.3
Bone Mineral Density
•AcomparativestudyofIMPLANONandanon-hormonal
IUDshowedthatbonedensityremainedunalteredover
2years,withnodetectabledifferencebetweenusersof
eachcontraceptivemethod.
Breast Milk
•AvailabledataindicatethatIMPLANONmaybeusedduring
lactationsinceitdoesnotinuencetheproductionor
thequalityofbreastmilk.However,itisimpor tanttobe
awarethatsmallamountsofetonogestrelareexcretedin
breastmilk.
•InclinicalstudiesuseofIMPLANONhadnoeffectonthe
productionorqualityofbreastmilkinnursingmothers.
Adverse Events
•Headache
•Weightincrease
•Acne
•Breastpain
•Irregularmenstruation
•Vaginalinfections
Contraindications
•UseofIMPLANONiscontraindicatedinpatientswith:
– Knownorsuspectedpregnancy
– Activevenousthromboembolicdisorder
– Presenceofhistoryoflivertumors
(benignormalignant)
– Presenceorhistoryofseverehepaticdiseaseaslong
asliverfunctionvalueshavenotreturnedtonormal
– Knownorsuspectedsex-steroidsensitivemalignancies
– Undiagnosedvaginalbleeding
– Hypersensitivitytotheactivesubstanceortoanyof
theexcipientsofIMPLANON
Drug Interactions
•Interactionscanoccurwithmedicinalproductsthat
inducemicrosomalenzymes,specicallycytochrome
P450enzymes,whichcanresultinincreasedclearance
ofsexhormones(eg,phenytoin,barbiturates,primidone,
bosentan,carbamazepine,rifampicin,andHIVmedication
[eg,ritonavir,nelnavir,nevirapine,efavirenz],andpossibly
alsooxcarbazepine,topiramate,felbamate,griseofulvin,and
theherbalremedySt.John’swort).
Womenontreatmentwithanyofthesedrugsshould
temporarilyuseabarriermethodinadditiontoIMPLANON.
Pleaserefertotheregulatory-approvedfullPrescribing
Informationforadditionalinteractions.
Pregnancy
•IMPLANONisnotindicatedduringpregnancy.Ifpregnancy
occursduringuseofIMPLANON,theimplantshouldbe
removed.
•Animalstudieshaveshownthatveryhighdosesof
progestagenicsubstancesmaycausemasculinizationof
femalefetuses.Extensiveepidemiologicalstudieshave
revealedneitheranincreasedriskofbirthdefectsin
childrenborntowomenwhousedoralcontraceptives
(OCs)priortopregnancy,norofateratogeniceffectwhen
OCswereinadvertentlyusedduringpregnancy.Although
thisprobablyappliestoallOCs,itisnotclearwhetherthis
isalsothecaseforIMPLANON.
KeyPointsforPatientCounseling
•Womenarelikelytohavechangesintheirmenstrual
bleedingpatternwithIMPLANON.Thesemayinclude
changesinbleedingfrequency,intensity,orduration;
however,thebleedingpatternexperiencedduringthe
rst3monthsisbroadlypredictiveoffuturebleeding
patternsformanywomen.
•Amenorrheawasreportedinabout1of5women
whileanother1of5womenreportedfrequentand/or
prolongedbleeding.
•Dysmenorrheatendedtoimprovewhileusing
IMPLANON.
•Appropriatecounselingmaymakebleedingchangesmore
acceptableforwomen.
•Keycounselingpointsinclude:
– Discussionofthelikelihoodofalterationsinbleedingpatterns
– Discussionoftherisks,benets,andpossiblesideeffects
•Explaintheinsertionandremovalprocedures;emphasize
thattheimplantshouldalwaysbepalpableandthatscars
orcomplicationsmayoccur.
•Ifpossible,providepatienteducationmaterials.
•Allowsufcienttimeforthepatienttoreviewthe
educationalmaterials,consideroptions,andaskquestions.
PAGE: 1References: 1–Graesslin2008,2–Blumenthal2008,3–Davies1993 PAGE: 2