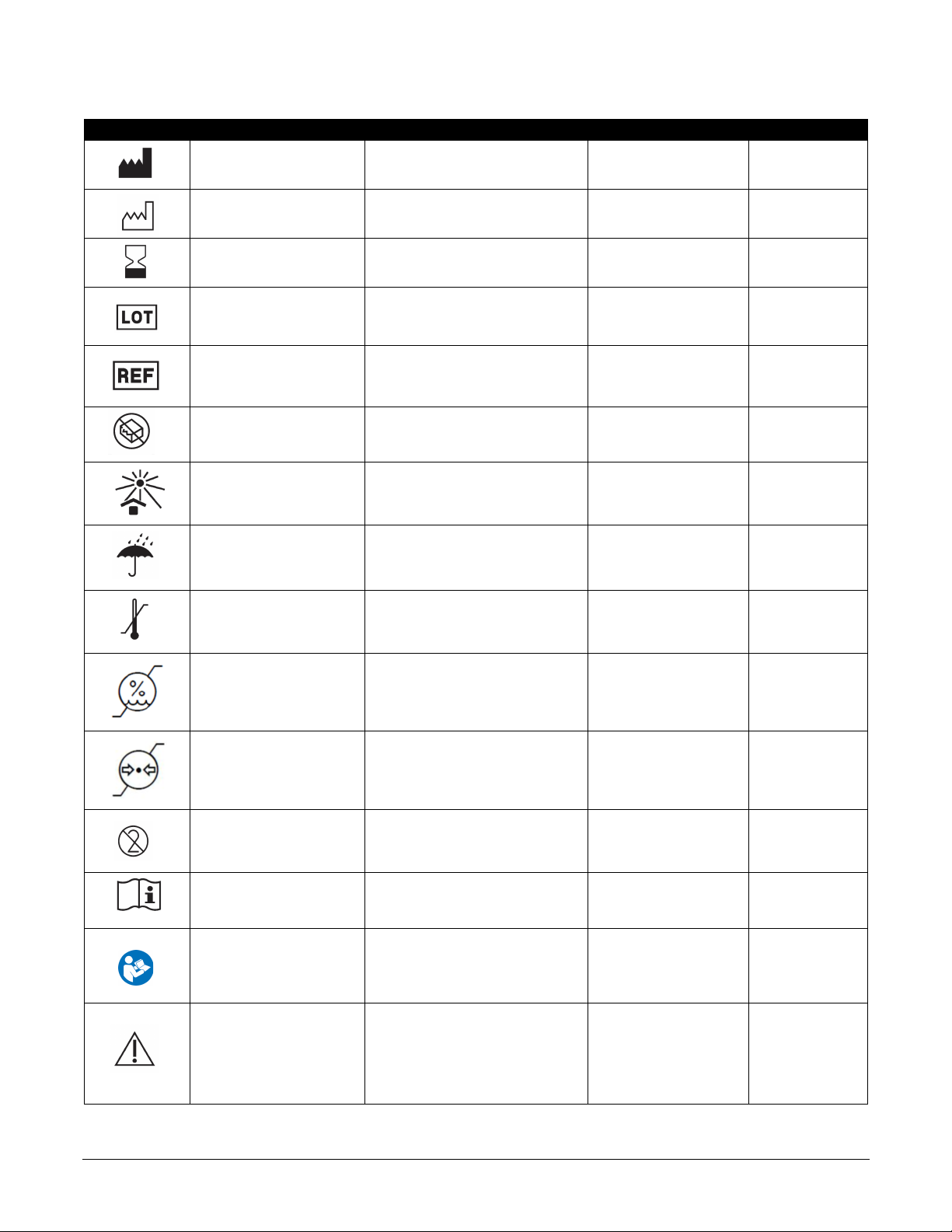
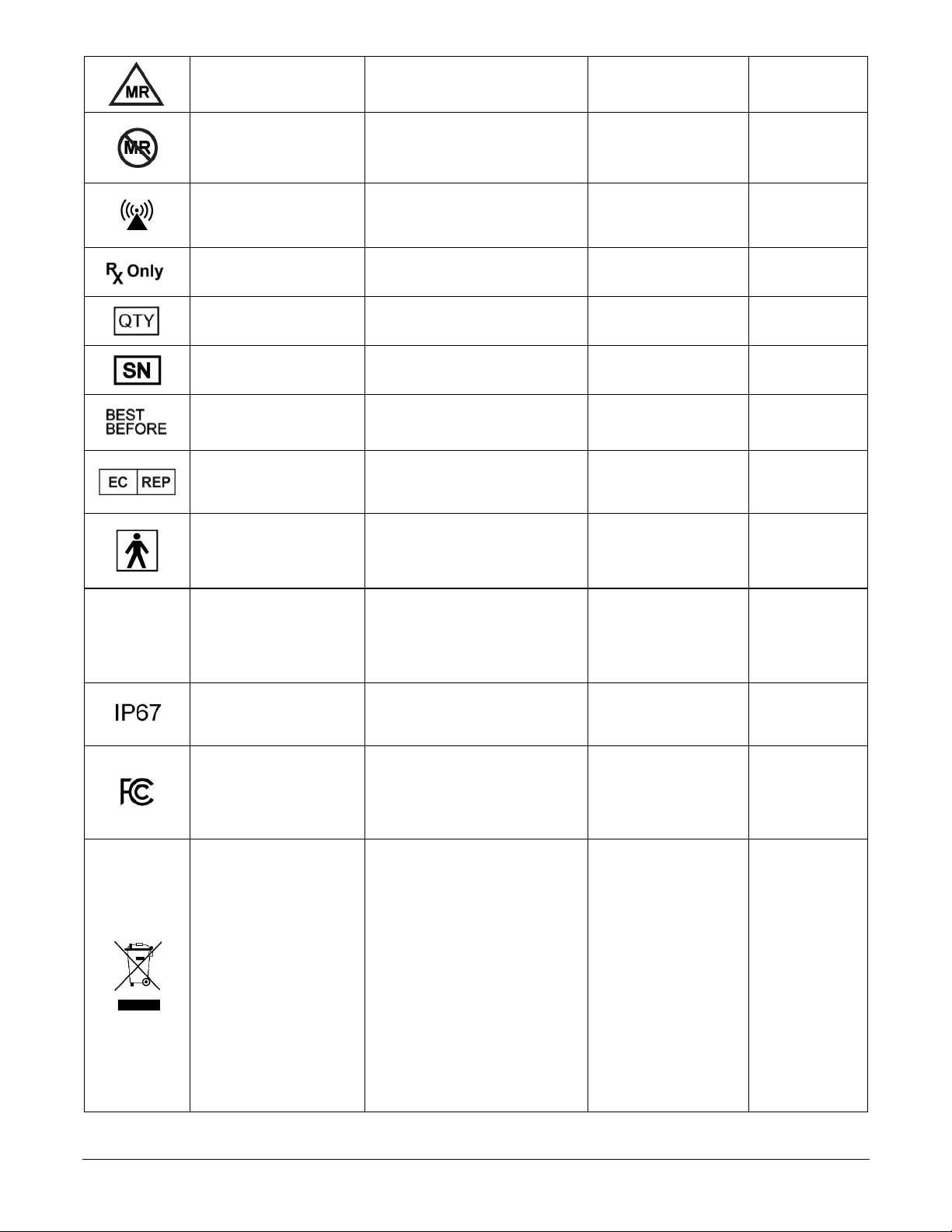
Nalu User’s Kit Instructions for Use PN: MA-000007 Rev C Page 9 of 44
Portable RF communications equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the Nalu Neurostimulation System. Otherwise,
performance degradation of the equipment might occur.
Machinery or Heavy Equipment—Machinery and heavy equipment (including vehicles) should not be operated
while using the Nalu Neurostimulation System. Malfunction of the System could result in the loss of body control,
body function, or a feeling that could render the user incapable of controlling the equipment.
Theft Detectors and Metal Screening Devices – Certain types of antitheft devices, such as those used at the
entrances/exits of department stores, libraries, and other public establishments, and/or airport security screening
devices may affect stimulation. If you are sensitive to low stimulation thresholds, you may experience a momentary
increase in perceived stimulation, which has been described as “uncomfortable” or “jolting”. Use caution when
approaching such a device and request assistance to bypass the device. If you must proceed through the device,
remove the Therapy Disc and proceed with caution, but be sure to move through the detector quickly.
Temperature Rise During Stimulation – During prolonged use of Therapy Disc the temperature of the device may
rise by 1°C above ambient temperature. If the Therapy Disc becomes uncomfortable remove the device from the
clip and discontinue use.
When you are considering additional medical tests or treatments, please share the following
Warnings with your clinician.
Active Implantable or Body-Worn Medical Devices—Safety has not been established for users who use the Nalu
Neurostimulation System with other active implantable or body-worn medical devices. Malfunction and/or damage
could occur to either system that could result in harm to the user or other people nearby
Magnetic Resonance Imaging (MRI)—MR Unsafe – For the Nalu Neurostimulation System, the only components
that are allowed into the MRI system room are the 40 cm Lead (Model 12001-040), the Nalu Anchor (Model 13001)
and the Nalu Implantable Pulse Generators (Model 11001-040, 11002-040, 11003-002, 11004-002).
All other components (i.e., the external component and programmer) are not permitted in the MRI system
room.
Magnetic resonance imaging (MRI) – MR Conditional – Prior to conducting or recommending an MRI
examination on a patient with the Nalu Neurostimulation System, it is important to read and understand the entire
section entitled, “MAGNETIC RESONANCE IMAGING (MRI) SAFETY INFORMATION” in the Table of Contents,
which pertains to performing an MRI examination safely in a patient.
These instructions apply only to the Nalu Neurostimulation System and do not apply to other products. If
you have any questions, please contact Nalu Medical or visit Nalu’s website <www.nalumed.com>.
The only Nalu Medical components that are labeled and approved as MR Conditional are the Lead (Model
12001-040), the Nalu Anchor (Model 13001) and the Nalu Implantable Pulse Generators (Model 11001-040,
11002-040, 11003-002, 11004-002). All other components are MR Unsafe.
Computed Tomography (CT) Scanning—Safety has not been established for CT scanning of users with a Nalu
Neurostimulation System. X-rays from the scan could cause unintended shocks or malfunctions of the System, and
may not be immediately detectable.
1. The CT operator should use CT scout views to determine if implanted medical devices are present and
their location relative to the programmed scan range. For CT procedures in which the device is in or
immediately adjacent to the programmed scan range, the operator should: