NeoLight SKYLIFE User manual

SKYLIFETM PHOTOTHERAPY SYSTEM
USER MANUAL
Website: www.theneolight.com
Address:275 N Gateway Drive, Suite #128 Phoenix, AZ 85034
Contact: support@theneolight.com or (480) 626-0304

1 INTRODUCTION 2
2 INTENDED USE 3
3 SKYLIFE SYSTEM COMPONENTS 3
4 EXPLANATION OF SYMBOLS 4
4.1 Warnings & Precauons 5
5 SKYLIFE OPERATING INSTRUCTIONS 6
5.1 Seng up the system 6
5.2 Preparing the baby for phototherapy 8
5.3 Administering phototherapy 9
5.4 Roune maintenance 11
6 CUSTOMER SERVICE AND MAINTENANCE 12
6.1 Customer service 12
6.2 Repair & Maintenance / Alarms 12
6.3 Calibraon 13
6.4 Labeling Standards 13
6.5 Operang condions 13
6.6 Transportaon and storage condions 14
6.7 Expected service life and disposal condions 14
6.8 Replacement parts informaon 14
6.9 Warranty 14
7 TECHNICAL SPECIFICATIONS 15
7.1 Electrical specicaons 15
7.2 Light Distribuon 15
7.3 Physical specicaons 15
7.4 Compliance declaraon 16
7.5 Stabilizaon me
7.6 Guidance & Manufacturer’s Declaraon
16
17
CONTENTS
500007, Rev P, Pg 1
1. INTRODUCTION
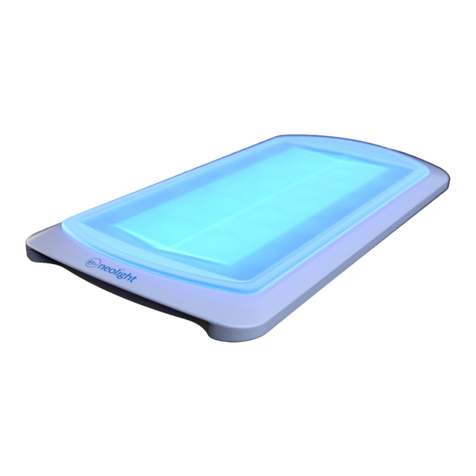
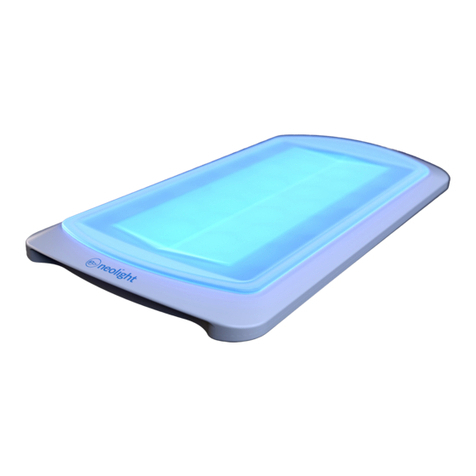
The Skylife Phototherapy System is a portable phototherapy device that delivers a narrow band of high-intensity
light via blue light eming diodes (LEDs) to provide treatment for neonatal unconjugated hyperbilirubinemia. The
Skylife system is designed to provide phototherapy treatment from underneath the baby minimizing interference
with other ongoing treatments. The Skylife device must be used within a paent bed, such as a bassinet, an open
crib, a warming table, or incubator and may be used in either hospital or home sengs.
The terms “neonatal” and “neonate” refer to a baby from birth through the rst 28 days of life. The Skylife deviceis
intended for use with all neonatal subpopulaons.
The system ulizes blue LEDs to achieve intensies from 25 µW/cm2/nm to >55 µW/cm²/nm*, eming light in a
narrow bandwidth between 430-475 nm. This light bandwidth corresponds to the spectral absorpon of light by
bilirubin, and is thus considered to be the most eecve for treatment 1,2, 3 . The Skylife system ulizes blue LEDs in
this range to achieve peak intensies between 25 to 35, 35 to 55, and over 55 μw/cm²/nm* at low, high, and very
high sengs.
The Skylife device greatly minimizes the risk of UV exposure typically seen with phototherapy treatment through
the use of Blue LEDs, as this light source does not emit signicant energy in the ultraviolet (UV) spectrum.
However, as with all phototherapy treatment, protecve eye masks must be used to protect the baby’s eyes from
blue light exposure.
Treatment mes can range from hours to weeks and should only be performed under the supervision of a licensed
praconer. Treatment exposures may be applied connuously 24 hours a day or as prescribed by the treang
physician. The Skylife device may be contraindicated for babies with rapidly rising bilirubin levels. Babies with
rapidly rising bilirubin levels must be monitored frequently and may require a more intensive therapy.
Read this User Manual carefully before using the device. Please pay careful aenon to the Safety
Informaon and Warnings throughout the manual.
Cauon: Federal law restricts this device to sale by or on the order of a praconer licensed by the
law of the State in which he/she pracces to use or order the use of the device.
This product is covered by one or more U.S. Patents.
1. Vreman, Hendrik J., et al. “Light-eming diodes: a novel light source for phototherapy.” Pediatric research 44.5 (1998): 804-809.
2. Lee, Kwang-Sun, and Lawrence M. Gartner. “Spectrophotometric characteriscs of bilirubin.” Pediatric research 10.9 (1976): 782-788.
3. Dixon, J. M., M. Taniguchi and J. S. Lindsey (2005), “PhotochemCAD 2. A Rened Program with Accompanying Spectral Databases for
Photochemical Calculaons, Photochem. Photobiol., 81, 212-213.
* Very High seng provides an average intensity of 56.3 μw/cm2/nm over the treatment area with peak intensity at 72.4 μw/cm2/nm.
500007, Rev P, Pg 2
2. INTENDED USE
The Skylife system is intended for the treatment of neonatal unconjugated hyperbilirubinemia. It is designed
to provide phototherapy treatment from underneath the baby. The Skylife unit must be used within a paent
bed, such as a bassinet, an open crib, a warming table or an incubator. The system can be used in a clinical
seng or in the home.
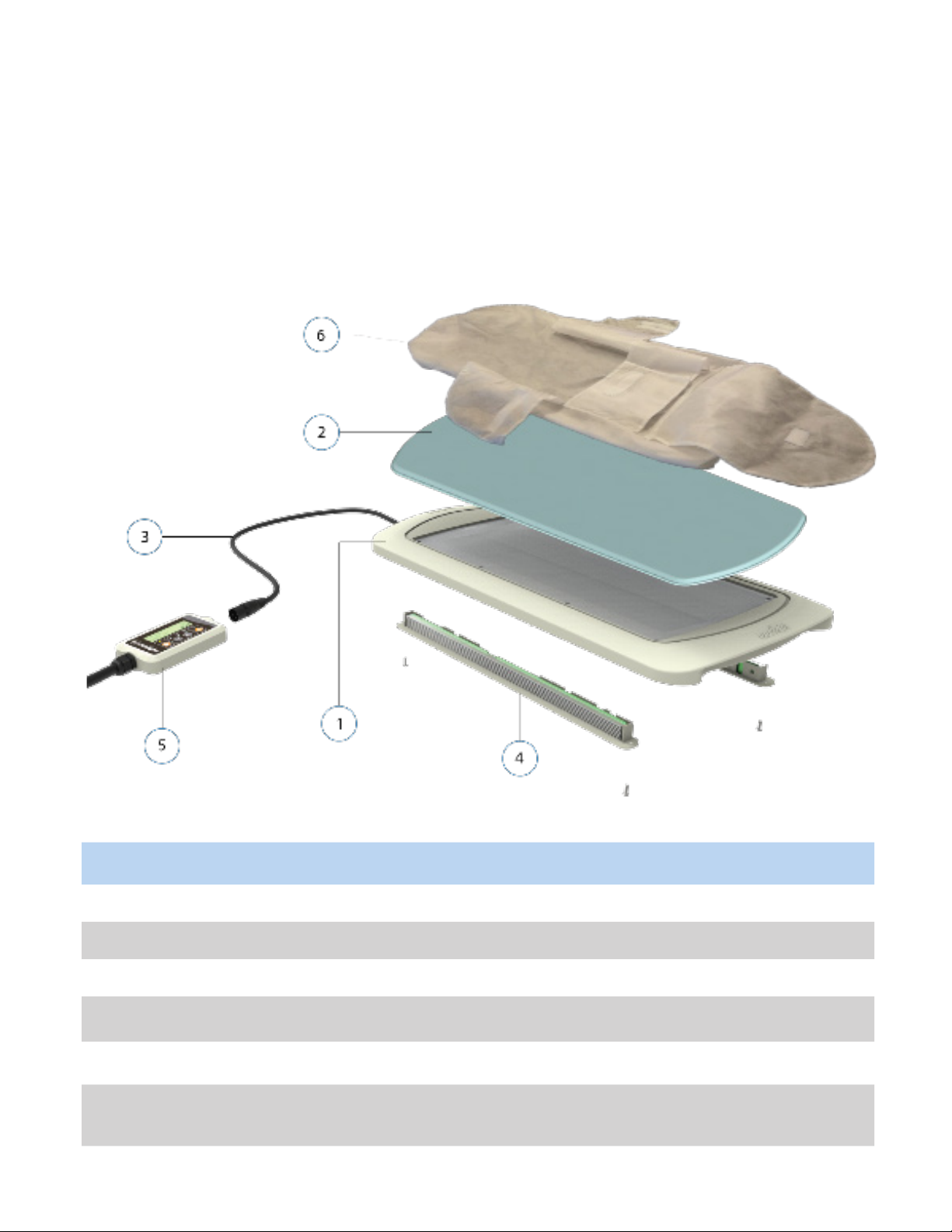
3. SYSTEM COMPONENTS
No. Part Name Descripon
1. Light Bed Top part of main device that emits blue light
2. GelMat Protecve cushion aached to the top of the bed
3. Light Bed Cable Connects device to Skylife controller
4. Light Modules Modules hold the light source inside the Light Bed
5. Controller Allows user to operate/control the device
6. Cloud Swaddle / Cloud
Swaddle Plus
Disposable swaddle that covers the device and ensures hygienic use
for baby
500007, Rev P, Pg 3
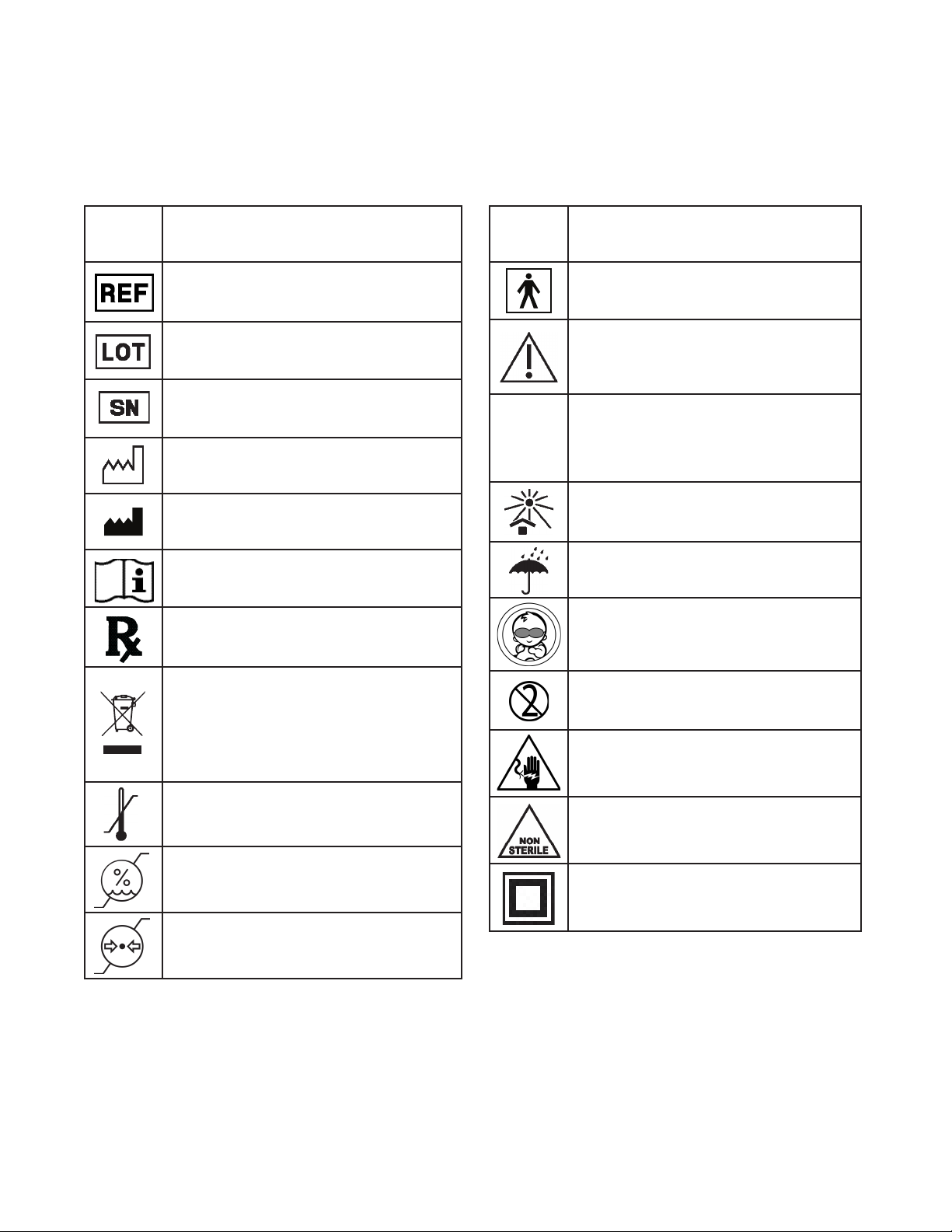
4. EXPLANATION OF SYMBOLS
The following symbols are used in this user manual, on the device packaging, on the device and
accessory labeling.
Symbol Descripon
Reference number; part number
Lot number
Serial number
Manufacturing date
Legal Manufacturer name and address
Follow instrucons for use
Prescripon only (USA)
Product contains electrical and electronic
equipment. User should not discard
this product along with other household
waste; it must be collected and treated
separately
Minimum and maximum operang and
storage temperature range
Minimum and maximum storage
humidity range
Minimum and maximum operang
atmospheric pressure range
Symbol Descripon
Type BF applied parts
Cauon
IP23
Protected electrical shock from touch
by hands greater than 12 millimeters.
Protected from water spray less than 60
degrees from vercal
Keep the device away from sunlight
Keep the device dry
Baby Eye Protecon Required
Single use only. Do not reuse.
Hazard of severe electric shock or burn
Non Sterile
Class II equipment
500007, Rev P, Pg 4

4.1 WARNINGS & PRECAUTIONS
1. Use the Skylife Phototherapy System only for its intended use as described in this manual.
2. Never operate the system if it has a damaged plug, damaged or frayed power cord or wires. Do not insert
anything into the end of the plug.
3. Always connect the device to a properly grounded outlet. Do not use an extension cord.
4. Always use the Skylifesystem in a crib, bassinet, incubator, or warmer where walls protect the baby from injury.
These environments must have a stable base that does not rock or p, as in the case of a rocking cradle.
5. Avoid using the device adjacent to or stacked on other equipment (except for incubators, warmers, bassinets, or
crib) as it could result in improper operaon.
6. Do not use the device in the presence of ammable substances such as anesthecs, cleaning agents and gases
that support combuson.
7. Do not disassemble the Skylife device unless you are a cered technician.
8. Do not use items such as blankets to cover the device.
9. Do not place the device where it can fall or be pulled into a tub, sink, or other liquid source.
10. Keep the Skylife controller in a locaon that is inaccessible to the baby.
11. Keep the Skylife controller and cords in a locaon away from toddlers, children and pets.
12. The Skylife Light Bed and GelMat must be used only with the Cloud Swaddle / Cloud Swaddle Plus provided. Any
other type of cover may cause a reducon in light intensity.
13. Baby eye protecon is required prior to turning on the Skylife unit.
14. If the user should experience discomfort from exposure to blue LED light, eye protecon is recommended
(yellow lenses) while operang the unit .
15. Baby should be wearing only a diaper and not otherwise wrapped or clothed during treatment.
16. Ensure baby is secured during treatment.
17. Ensure that device cords and any other equipment cords are outside treatment area and do not pose an
entanglement, strangulaon, or tripping hazard.
18. Do not operate the Skylife unit in temperatures above 37oC (98.6oF). Keep the device away from heated
surfaces, heaters, and other heat sources (like replaces or warming blankets).
19. Do not use the device while bathing or feeding the baby.
20. During phototherapy, the baby’s water balance may become disturbed. Before and during treatment, make sure
the baby is properly hydrated and that his or her body temperature is maintained.
21. During phototherapy, monitor baby’s bilirubin levels according to your praconer’s recommendaons.
22. The Skylife device should be turned o prior to evaluang baby’s skin color, as lighng will eect visual color
evaluaon. Parents should contact their medical praconer if needed.
23. Turn o and unplug the unit during device servicing.
24. If the Light Bed is exposed to liquid (falling into water or uid is spilled on the device), immediately unplug the
unit from the power source prior to taking any other acon. Disconnue use of the device immediately.
25. Store the device in dry locaon away from direct sunlight. Avoid exposure to dust, lint or other parculates.
26. Do not place heavy objects on the Light Bed. This can damage the panel and may aect light output.
27. Do not place sharp objects on any element of the deviceas it may cause damage.
28. Do not drop the device. If the system is dropped, contact your hospital technician or the manufacturer for
further informaon prior to resuming use of the Skylife unit.
29. Ensure that the device is inaccessible to children or pets when not in use.
30. Handle GelMat with care. Do not stretch, twist, or fold the GelMat.
31. Keep sharp objects away from GelMat.
32. Do not clean the Light Bed top surface with any liquids. Use dry cloth wipes only on the cloudy area
of the Light Bed.
33. Due to photo eects, drugs should not be stored in treatment area.
34. Not for use with babies greater than 10kg (22 lbs).
35. Do not use the Skylife system without GelMat and Cloud Swaddle, or place baby directly on the Light Bed.
36. Always ensure to rmly secure the neonate’s arms, legs, and body using use Cloud Swaddle Plus.
500007, Rev P, Pg 5
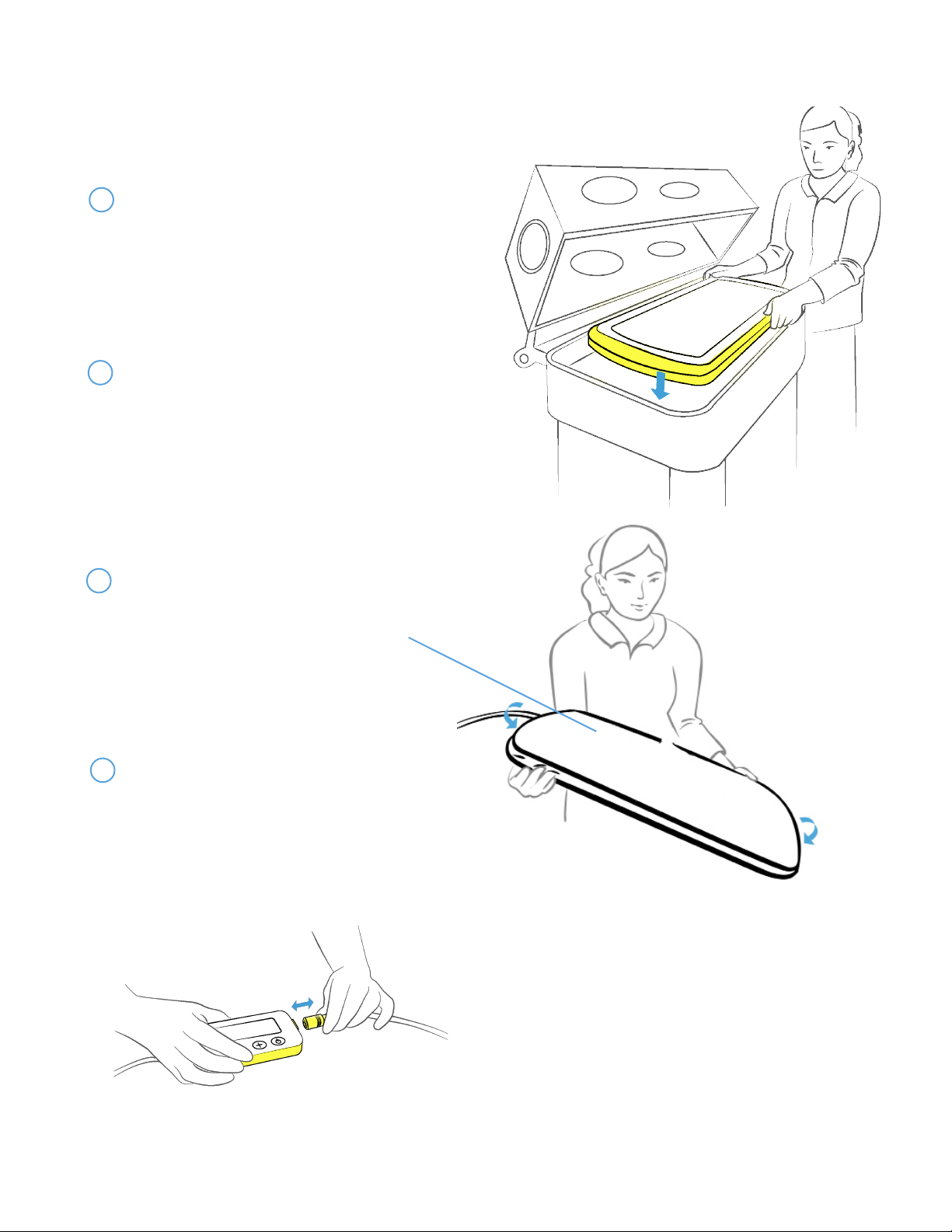
5. SKYLIFE OPERATING INSTRUCTIONS
(Steps 1 - 6 are applicable in both home and hospital sengs.)
5.1 SETTING UP THE SYSTEM
1 Place the Skylife device in a locaon with walls
(e.g. bassinet, warmer, crib, or incubator).
Cauon: Do not allow items such as blankets to cover
the outside edges of the device.
2Visually inspect Light Bed and GelMat
for damage or wear. If GelMat becomes
damaged, torn, or develops a yellowish
discoloraon, refer to secon 6.2, steps
#1 - 4 for replacement informaon. A home
user should contact the system provider for
replacement.
Place the Cloud Swaddle on the device
over the GelMat. Secure the Cloud Swaddle
to the device using the corner pockets like a
ed bed sheet.
3
Aach the Controller and Power Supply
to the device and place the Controller
outside the enclosing walls of the treatment
area. Ensure that the cord does not pose a
hazard to the baby. Plug the Power Supply
into the wall outlet.
4
500007, Rev P, Pg 6
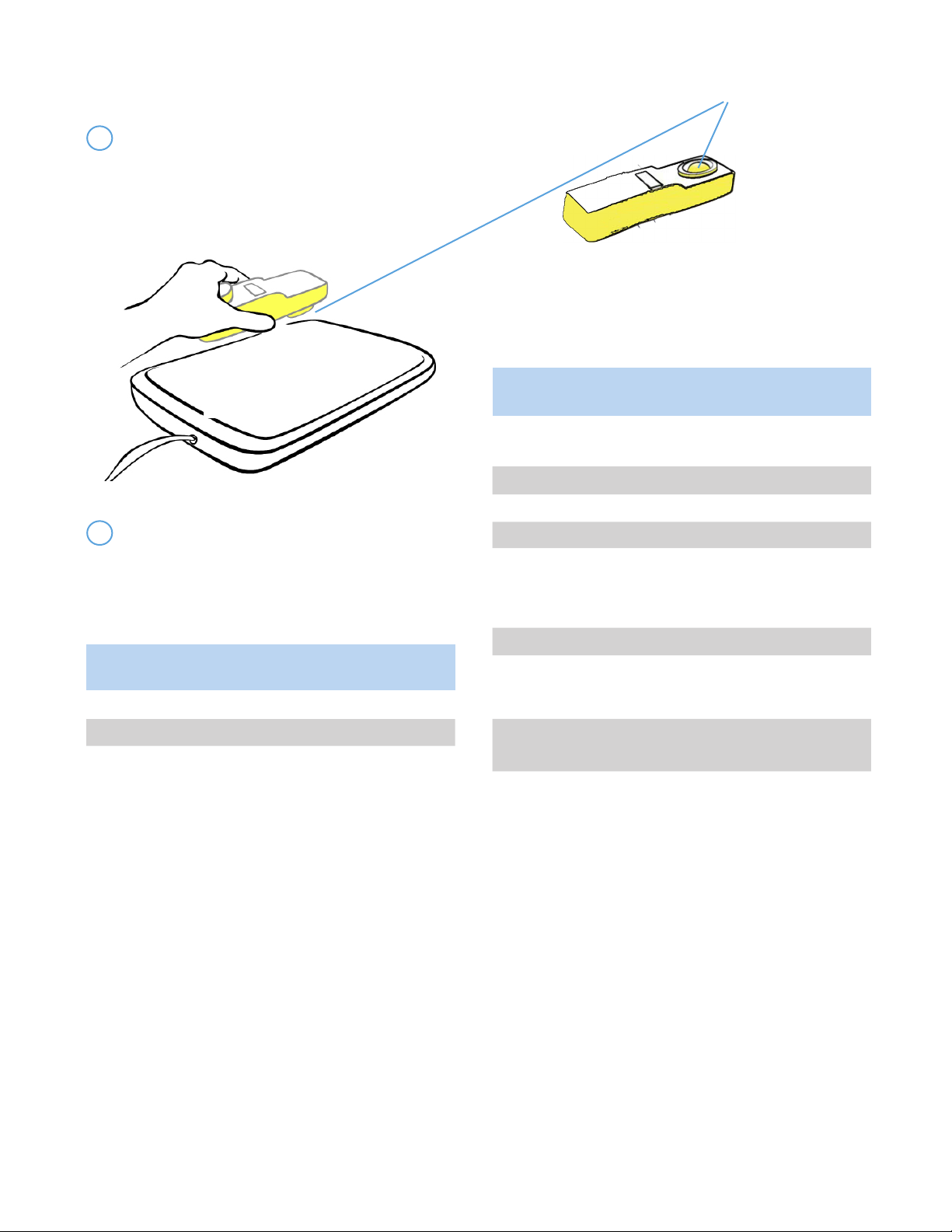
Light Sensor
500007, Rev P, Pg 7
(Steps 5 - 6 are applicable in a hospital seng only, including
technical references on this page.)
5 Prior to placing the baby on the device, turn on the
device to the desired treatment level(s). To check the
light intensity, follow instuonal pracces.
The device has been calibrated to deliver the
following light Intensity levels
Low 30 ± 5 µW/cm²/nm
High 45 ± 10 µW/cm²/nm
Very High >55 µW/cm²/nm*
Cauon: If device does not meet the desired intensity range for treatment, replace GelMat
and repeat setup. If device sll fails to meet the required levels contact Customer Service.
Warning: Varying ambient lighng (including exposure to sunlight and other
photoradiaon sources) and varying temperature condions may aect paent response to
treatment, monitor paent closely.
Recommended Spectrophotometer Specicaons
Recommended Model Ohmeda Medical
BiliBlanket Meter
Spectral Response 400-520 nm
Center Wavelength 450 nm
Bandwidth 60 nm
Light Acceptance Angle
Cosine Characteriscs
±2% @ 30° angle
±7% @ 60° angle
±25% @ 80° angle
Receptor Type Silicon Photocell
Measuring Funcon Spectral Irradiance
μW•cm-2•nm-1
Accuracy Within ± 3% of reading ±1
digit in the last posion
6 Measurements should be taken on the surface of
the GelMat. The Cloud Swaddle must be on the device
when taking readings and be sure to focus the light
sensor towards the GelMat.
* Very High seng provides an average intensity of 56.3 μw/cm2/nm over the treatment area with peak intensity at 72.4 μw/cm2/nm.

5.2 PREPARING BABY FOR PHOTOTHERAPY
1Before starng treatment, always shield the
baby’s eyes with a protecve eye mask
designed for use during phototherapy. During
phototherapy treatment, regularly remove the
coverings according to hospital policy to assess
the baby’s eyes for signs of irritaon or infecon.
Important! Eye Protecon: Do not look directly
into the blue light for a prolonged period of me.
3 To power on the device, press and
hold the power buon on the controller
unl you hear a beep and the lights
come on.
2 Posion the baby on the Cloud Swaddle.
Ensure that only the Cloud Swaddle is directly
under the baby. Do not use blankets, swaddles,
bumpers, etc. to cover the device.
Important: During treatment, always ensure
to completely secure the neonate using Cloud
Swaddle Plus
500007, Rev P, Pg 8
The device has been calibrated to deliver the
following light Intensity levels:
Low 30 ± 5 µW/cm²/nm
High 45 ± 10 µW/cm²/nm
Very High >55 µW/cm²/nm*
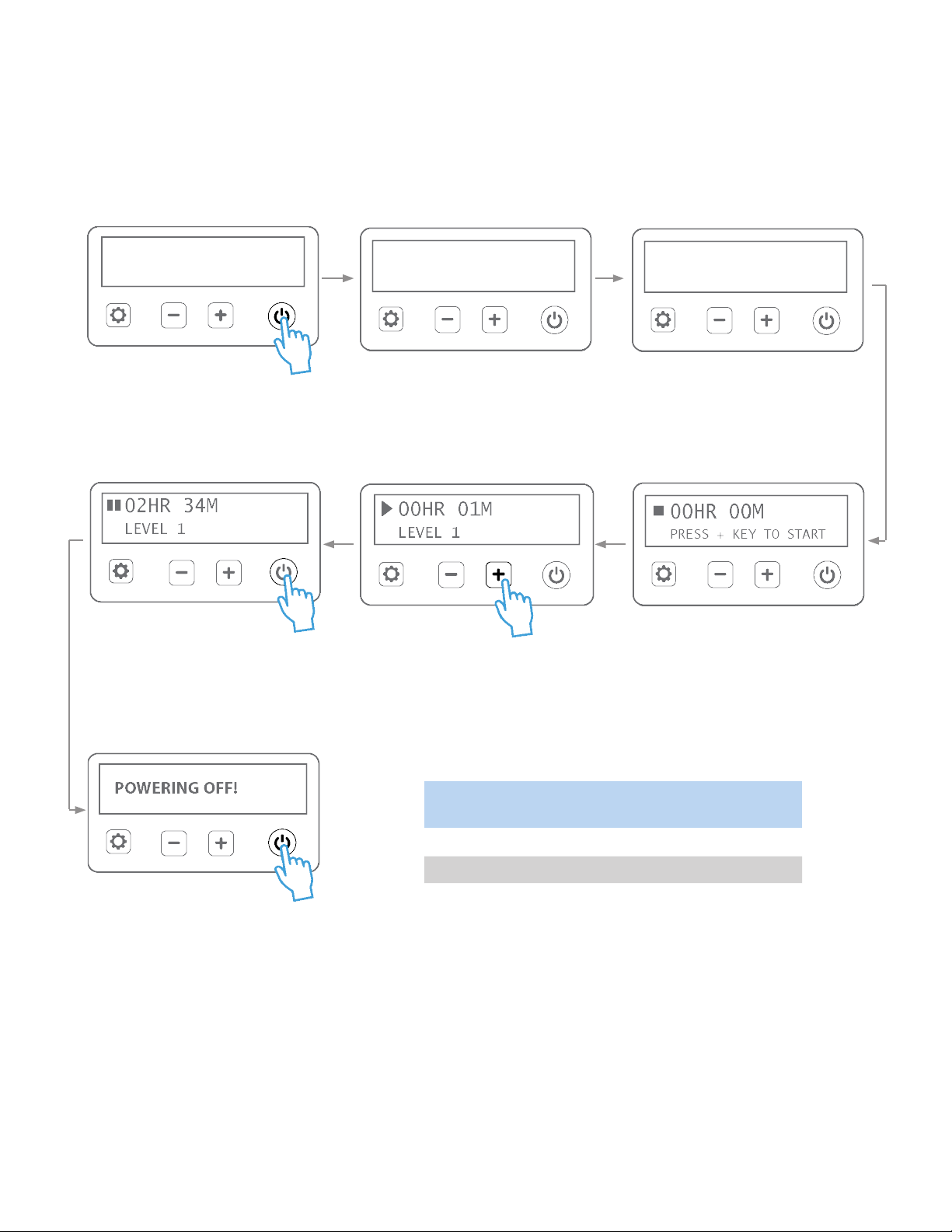
5.3 ADMINISTERING PHOTOTHERAPY TREATMENT
Normal use cycle:
Press and hold power buon unl a
beep is heard to start device. NOTE:
Soware version may dier than
version displayed above.
The device is now ready for use.
Press ‘+’ buon once to begin treatment.
Use ‘+’ and ‘-’ buons to increase or
decrease light intensity. LED indicators will
glow to show corresponding levels. Keep
device at a seng recommended by the
treang physician.
To pause treatment, press power buon
once. To resume treatment, press power
buon once again.
Press hold the power buon for a longer
duraon to power OFF the device.
* Actual display may vary based on soware revisions.
** LED lifeme refers to the number of hours the LED modules have been used to date.
Once baby is secure, turn on the device and use the controller to administer therapy. Follow the steps below and
set light intensity level as directed by physician to achieve desired results*.
SELF TEST PASSED LED LIFETIME
2,435 HRS
System performs self-check and buzzer
beeps if passed. In case of failure, a sys-
tem alarm is generated, refer to secon
for 6.2 for more informaon.
System will display LED lifeme**.
Verify that the me is less than 25,000
hrs.
NEOLIGHT SKYLIFE PHOTOTHERAPY
VERSION 1.0
500007, Rev P, Pg 9
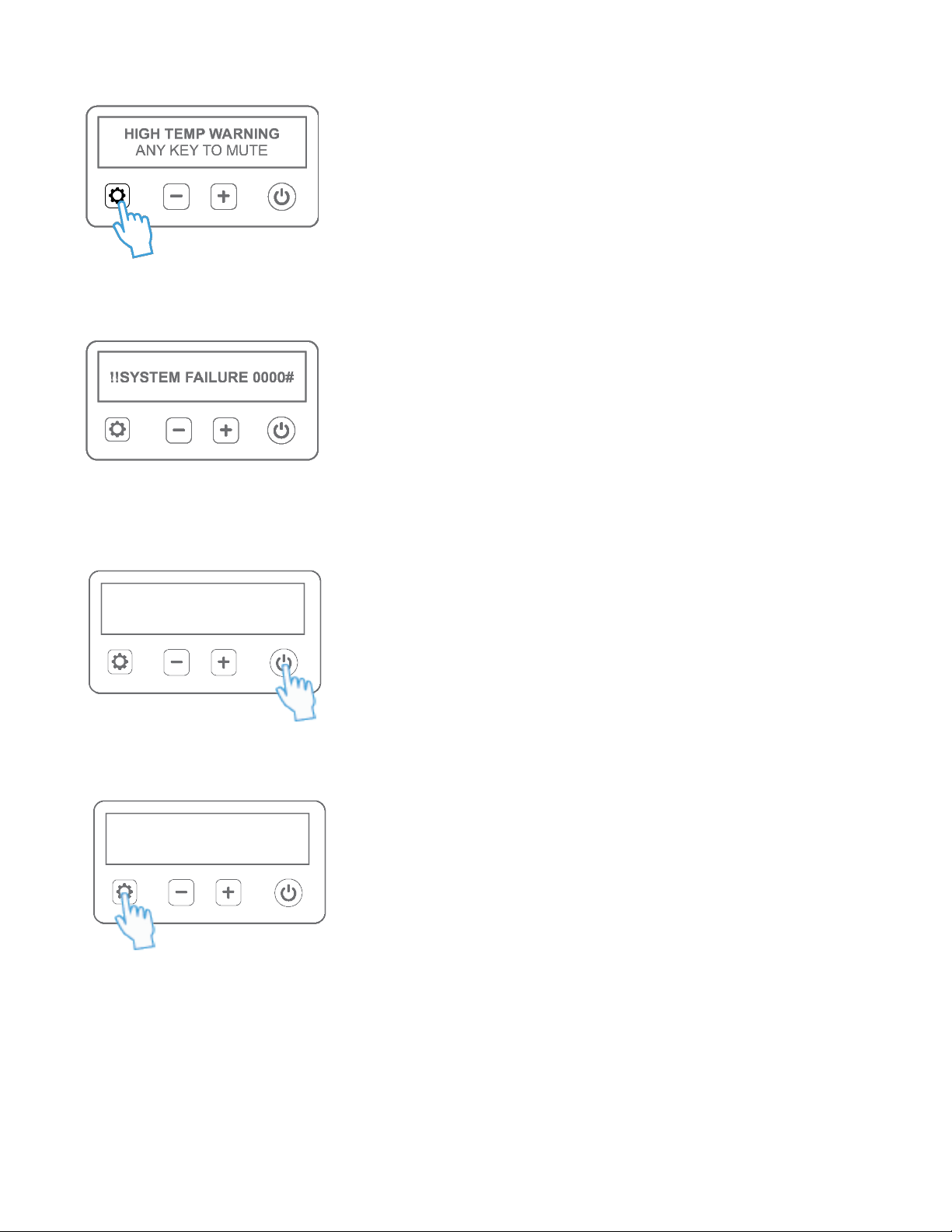
High Temperature Alarm Condion:
If the system temperature increases beyond a certain threshold, the High Temperature
Alarm will sound and LED indicators on the controller will ash. Press any buon except
power buon to mute the alarm. Check if the air vents on the underside of the device
are blocked, remove any items that may be blocking air vents. Resume treatment.
If high temperature condion persists, power OFF the device and remove the baby from
the Skylife device. Contact treang physician, technician, or manufacturer.
System Failure Condion:
If any part of the device fails to perform as expected, a “System Failure’ alarm will sound,
LED indicators on controller will ash and the device will shut OFF automacally. Restart
the device to see if it works. If the device shows the same error message or fails to power
on, contact treang physician, hospital technician, or manufacturer.
Sengs:
Press Sengs buon to view the system sengs. The Sengs will display following
informaon:
• LED Lifeme**
• Version Number
Only a cered technician may change these sengs
Accidental Power Disconnecon:
If the power cable is accidentally unplugged from the wall socket, plug in the power
cable back into the socket. The system will display a count down prior to automacally
connuing treatment. To pause treatment, press the power buon . To disconnue
treatment, press and hold the power buon.
Cauon: The system will automacally restart treatment within 10 seconds of power
applicaon.
POWER INTERRUPTED
RESUMING TREATMENT IN 09 SEC
LED LIFETIME
VERSION #
** LED lifeme refers to the number of hours the LED modules have been used to date. 500007, Rev P, Pg 10

Important! User Safety: Sensive individuals may experience headache, nausea or mild vergo in case of prolonged
exposure to illuminated surface. Using the Skylife device in a well-lit area or wearing glasses with yellow lenses can
alleviate potenal eects.
Monitor the baby during treatment.
Important! Baby Monitoring: Regular monitoring during treatment is recommended. Use the following guidelines:
• Measure the baby’s bilirubin level periodically during treatment per instuonal procedures or
treang physician’s instrucons.
• Turn o the treatment when checking the baby’s condion and assessing skin color.
• Follow standard procedures for monitoring the baby’s temperature and vital signs.
• Verify that the baby’s eyes are protected and free of infecon per instuonal procedures or
treang physician’s instrucons.
When treatment is completed, unplug the power supply and remove the device from the therapy area. Follow
proper cleaning procedures according to your instuons guidelines before storing the device.
5.4 ROUTINE MAINTENANCE
PRIOR TO TREATMENT
1. Ensure the unit is unplugged from the Power
Supply.
2. Inspect the GelMat for signs of wear or damage.
Replace worn or damaged GelMat prior to
treatment.
3. Place new Cloud Swaddle (Plus) on device.
Cauon: Do not use damp wipes or liquids of any
kind on the light eming area as this may damage
the device. Do not soak or spray water on the
device.
DURING TREATMENT
Replace the Cloud Swaddle every 24 hours at a minimum.
Should Cloud Swaddle become soiled for any reason:
1. Turn o the device and unplug the power cord from
outlet.
2. Remove and discard Cloud Swaddle following
instuon pracce.
3. Visually inspect GelMat and Light Bed for soil or liquid.
Clean as required per instuonal pracces or as
instructed below.
4. Allow the system to fully dry before connuing
treatment.
CLEANING
The Skylife system consists of four (4) components: Light Bed with Controller, GelMat (maress), Cloud Swaddle
(disposable maress cover), and the Power Supply. Only three of the four components are designed to be reused,
the Light Bed, GelMat, and Power Supply. The Cloud Swaddle is disposable/single use only.
Cloud Swaddle & GelMat
The Cloud Swaddle protects the GelMat (maress), and Light Bed from contaminaon during use. In the event
that bodily uids soak through the Cloud Swaddle to the GelMat, the GelMat may require cleaning. Do not remove
GelMat during cleaning.
Cleaning and Disinfecon Procedure
1. Unplug the device. Always turn o and disconnect the device from the power outlet during cleaning.
2. Remove Cloud Swaddle from device and discard. Make sure to replace Cloud Swaddle between paents,
whenever soiled, or every 24 hrs, whichever occurs rst.
3. Using recommended, pre-moistened disinfecon wipes (such as Super Sani Cloth®), remove all visible
contaminaon from the GelMat and verify under normal lighng. Do not remove GelMat during cleaning.
4. Aer all visible contaminaon has been removed, use disinfecon wipes to thoroughly wet the exposed surfaces
of the GelMat and exposed edges of the Light Bed.
5. Ensure that the surface remains visibly wet for 3 minutes at room temperature (68°F/20°C), or as specied by
product instrucons. Use addional wipes as needed to maintain a visibly wet surface.
6. Allow the surface to dry.
7. Follow this by using a clean damp cloth moistened only with clean water to rinse the same exposed surfaces.
Repeat this at least 2 more mes with a clean damp cloth or unl any remaining chemical residues are removed.
8. If the device is determined not to be visibly clean aer these steps, repeat the cleaning process or do not use
the device.
Professional and Home Usage
For healthcare and home use, make sure to use the disposable Cloud Swaddle according to the direcons above. The
Skylife device is to be cleaned using standard cleaning cloths (such as Super Sani Cloth®) prior to use. You may clean
the non-body contact areas such as the Light Bed, GelMat, controller, and power supply of the Skylife™ system using
standard cleaning wipes (such as Super Sani Cloth®).
If the unit is cleaned in a healthcare seng, make sure to refer to the instuonal cleaning procedures and
requirements for cleaning the non-body contact areas such as the Light Bed, GelMat, controller, and power supply.
Cauon: In either healthcare or home use seng, do not allow liquids to seep into the Light Bed housing. Do not
use strong acidic and alkaline soluons, and do not autoclave or gas sterilize the Skylife™ system. As menoned
above, always turn o the device and disconnect it from the power outlet during cleaning before reuse.
500007, Rev P, Pg 11
6. CUSTOMER SERVICE & MAINTENANCE
6.1 CUSTOMER SERVICE
Please contact Customer Service if you need assistance seng up, using, or maintaining your Skylife unit(s) or to
report any unexpected operaon or events.
When returning any products, please include your name, address, phone number, and Return Material
Authorizaon (RMA) number provided by Customer Service.
All product returns should be mailed to:
NeoLight LLC.
An: RMA # ________
275 N Gateway Drive
Suite #128,
Phoenix, AZ 85034
6.2 REPAIR & MAINTENANCE
• Please contact NeoLight Customer Service in case of any damage or failure of the Skylife system.
• Do not aempt to repair any part of your Skylife unit(s). Never dismantle the system due to risk of electric
shock. NeoLight LLC declines all responsibilies for any damages or consequences resulng from unauthorized
aempts to open, modify, or repair the device.
• The following components of the Skylife system must be replaced as directed for proper funcon of the system:
Part Name Part Number Recommended Replacement Time
LED Light Modules (pair) NL-SK-3 25,000 hours of use as indicated on the
controller
GelMat (Pack of 1) NL-SK-2-212
Every 1,500 hours of treatment, or 6 calendar
months, or if Light Intensity become reduced
below calibrated levels, whichever occurs rst.
Cloud Swaddle (Pack of 5) NL-SK-5 24 hours of use or if soiled
Cloud Swaddle Plus (Pack of 5) NL-SK-8 24 hours of use or if soiled
• Follow appropriate steps in response to system alarms. Always contact the treang physician in the event that
treatment with the Skylife device must be disconnued.
• The light intensity output of the the Skylife device is calibrated at NeoLight prior to shipment. For home use,
follow instrucons provided by care provider regarding light intensity measurements. For hospital use, it is
recommended to follow instuonal pracces or check light intensity prior to use with each paent.
GelMat
1. GelMat should be replaced if damage has been
observed or if it becomes discolored.
2. GelMat should only be replaced by a biomed
technician or other medical professional.
If a new GelMat is needed, contact NeoLight
Customer Service at (480) 626-0304
3. If installing a new GelMat, remove protecve liners from
the adhesive strips on the back of the GelMat.
4. Place GelMat on the Light Bed in the center of the device.
500007, Rev P, Pg 12
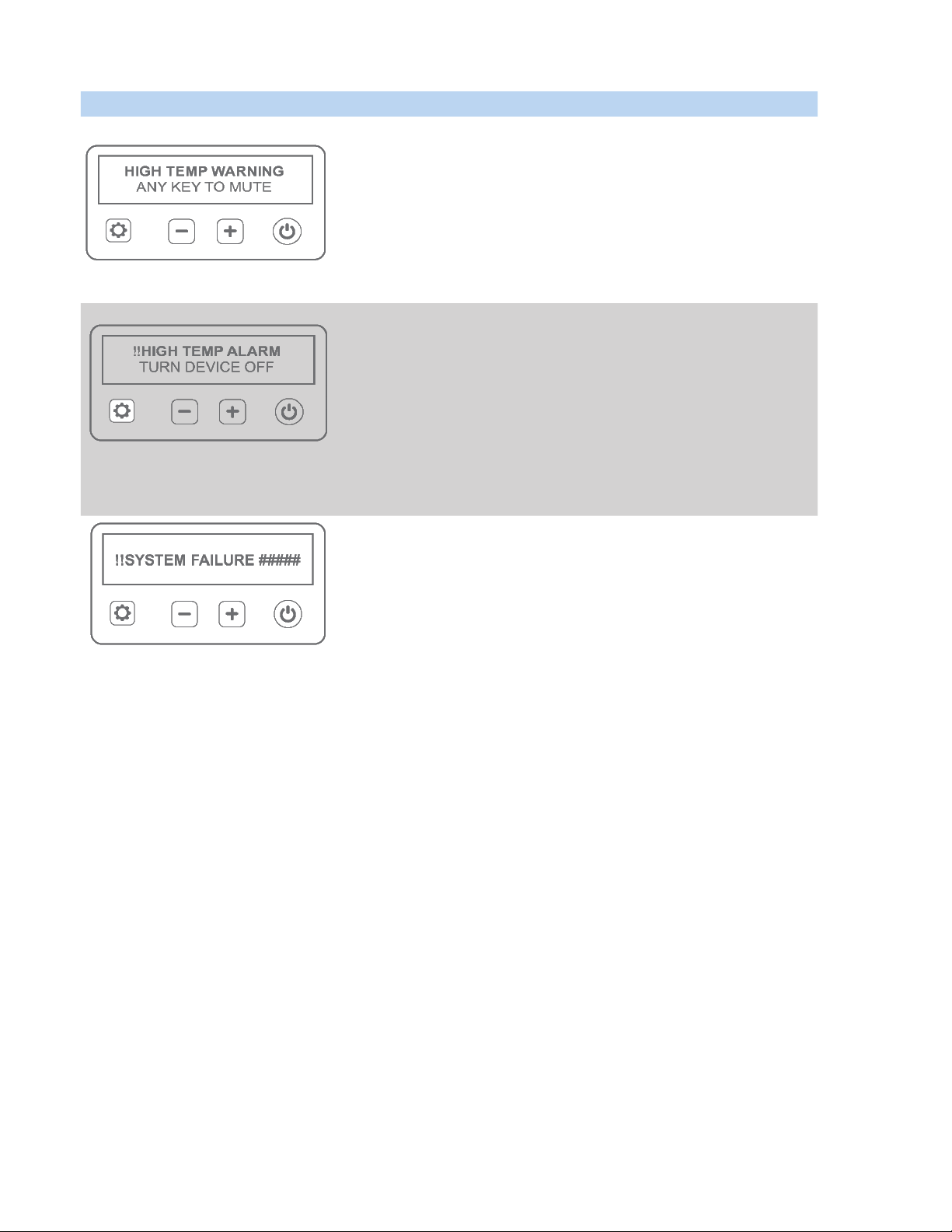
Displayed Warnings & Alarms Acon
Press any buon on the keypad to acknowledge the warning.
Verify that the vents are not blocked or covered, remove any obstrucons.
Treatment may connue unless alarm repeats.
If alarm repeats, disconnue treatment and contact NeoLight Customer Service.
Consult with treang physician for further treatment opons.
Press Power buon to turn o device and suspend treatment for a
minimum of 20 minutes to allow device to cool.
Verify that the vents are not blocked or covered, remove any obstrucons.
Treatment may connue unless alarm repeats. If alarm repeats,
disconnue treatment and contact NeoLight Customer Service.
Consult with treang physician for further treatment opons.
Contact NeoLight Customer Service for troubleshoong.
6.3 CALIBRATION
The Skylife device has been calibrated to Z540 STANDARD TRACEABLE CALIBRATION.
No calibraon is required by the user.
6.4 LABELING STANDARDS
Skylife device labels have been designed to ISO 15223-1.
6.5 OPERATING CONDITIONS
The Skylife system should be operated in temperatures between 50°F and 98.6°F (10°C and 37°C),
atmospheric pressures between 50 and 106 kPa (7.25psi to 15.37psi), and relave humidity between 15% and
90% RH.
500007, Rev P, Pg 13

6.8 REPLACEMENT PARTS ORDERING INFORMATION
To order replacement parts for the Skylife unit, contact NeoLight Customer Service.
No. Part Name Part Number
1. LED Light Modules (pair) NL-SK-3
2. GelMat (Pack of 1) NL-SK-2-212
3. Cloud Swaddle (Pack of 5) NL-SK-5
4. Cloud Swaddle Plus (Pack of 5) NL-SK-8
Important! Improper Operaon: Use of accessories, cables and replacement parts other than those supplied
by Neolight can increase electromagnec emissions and decrease electromagnec immunity resulng in
improper operaon of the system. Follow instrucons supplied with parts to replace them.
6.9 WARRANTY
NeoLight oers a warranty of 1 year for the Skylife system. In addion, Neolight warrants the light modules for
25,000 hours*. Should your Skylife unit develop a fault within the warranty period, NeoLight will replace your
Skylife device free of charge, provided the system:
• has been used for its intended purpose by the original owner and in the manner described in this manual.
• has not been connected to an unsuitable power source.
• has not been subjected to misuse or neglect.
• has not been modied or repaired by any unauthorized pares without prior consent from NeoLight.
*Actual results may vary
6.6 TRANSPORTATION AND STORAGE
The Skylife device should be transported and stored in temperatures between -13°F and 122°F (-25°C and 50°C),
atmospheric pressures between 50 and 106 kPa (7.25psi to 15.37psi) and relave humidity between 10% and 93% RH.
6.7 EXPECTED SERVICE LIFE AND DISPOSAL
The Skylife device is warranted to perform as expected for at least 1 year of normal use.
The Skylife device is a piece of electronic equipment and may include substances that can damage the environment.
DO NOT dispose of the device in municipal waste. Please deliver the device to a suitable collecon point for recycling
of electronic equipment in accordance with local regulaons.
500007, Rev P, Pg 14
7. TECHNICAL SPECIFICATIONS*
7.1 ELECTRICAL
AC Power 90-264 VAC, 50-60 Hz, 1.0A/115V- 0.5A/230V
Type of Protecon Against Electrical Shock Class II Equipment
Degree of Protecon Against Electrical Shock Type BF Applied Part
Degree of Protecon Against Ingress of Water IP23
Mode of Operaon Connuous
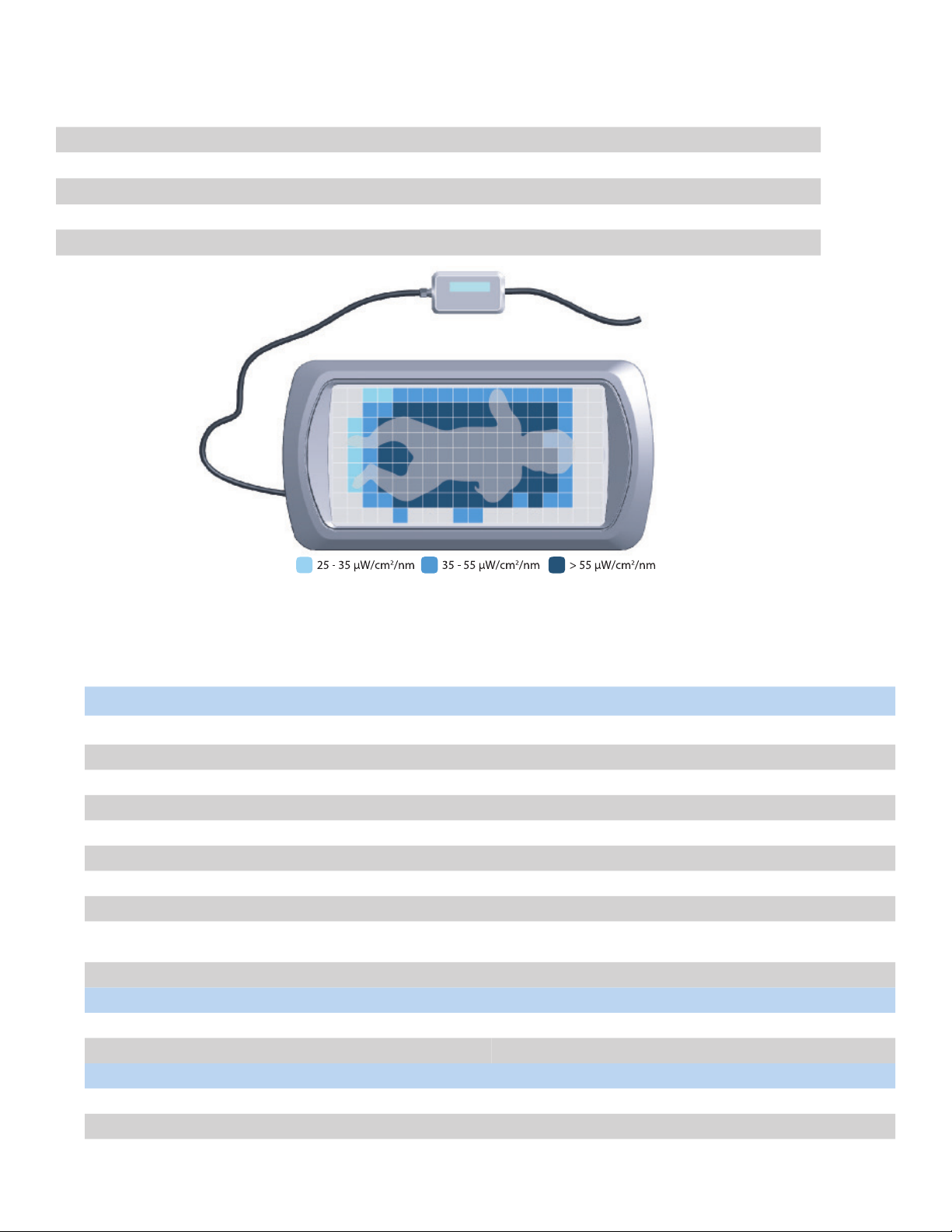
7.2 LIGHT DISTRIBUTION
Light Intensity distribuon map on Bed (with GelMat and Cloud Swaddle). Maximum intensity in the center.
The rao of minimum to maximum measured intensity across the eecve surface area is >0.4 (avg. 0.79).
(The Cloud Swaddle aenuates an average of 0.4 μW/cm²/nm.)
7.3 PHYSICAL
Physical Specicaons
Device Dimensions 23.94” L x 12.25” W x 1.91” H (60.81 x 31.12 x 4.85 cm)
Device Weight <11lbs (5 kg)
Paent Area 21.41” L x 10.24” W (54.38 x 26 cm)
Eecve Surface Area 119in2 (769 cm2)
Light Distribuon Rao (min to max) >0.4 (avg. 0.79)
Controller Dimensions 4.75” L x 2.75” W x 0.89” H (12.07 x 7 x 2.26 cm)
Controller Weight <0.5lbs (0.23 kg)
Length of device cord to controller 20” (50.8 cm)
Length of connecng cord between controller and
power adapter 53” (134.62 cm)
Length of power cord from adapter to plug 66.5” (168.91 cm)
Storage and Transportaon Condions
Temperature -25oC to +50oC (-13oF to 122oF)
Relave Humidity 10%-93% RH Non-condensing
Operang Condions
Temperature 10oC to 37oC (50oF to 98.6oF)
Relave Humidity 15%-90% RH Non-condensing
500007, Rev P, Pg 15*Specicaons subject to change without noce.

7.4 COMPLIANCE DECLARATION
IEC 60601-1: 2005, 3rd Edion
ANSI/AAMI ES60601-1
Medical electrical equipment - Part 1: General requirements for
basic safety and essenal performance
IEC 60601-1-2: 2014, 4th Edion Medical electrical equipment - Part 1-2: General requirements
for basic safety and essenal performance - Collateral Standard:
Electromagnec Compability - Requirements and Tests
IEC 60601-1-6: 2010, 3rd Edion Medical electrical equipment - Part 1-6: General requirements
for safety - Collateral Standard: Usability
IEC 60601-1-11: 2015 Medical electrical equipment - Part 1-11: Collateral Standard:
Requirements for medical electrical equipment and medical
electrical equipment used in the home healthcare environment
IEC 60601-2-50: 2012, 2nd Edion Medical electrical equipment - Part 2-50: Parcular requirements
for the safety of infant phototherapy equipment
IEC 60601-1-8: 2006 Medical electrical equipment — Part 1-8: General requirements
for basic safety and essenal performance — Collateral standard:
General requirements, tests and guidance for alarm systems in
medical electrical equipment and medical electrical systems
IEC 62304: 2006 Medical device soware — Soware life cycle processes
7.5 STABILIZATION TIME
The Skylife Phototherapy System does not require a pre-aging me. It is ready for use immediately aer it is
turned on the inial me.
The Skylife Phototherapy System does not require a stabilizaon period. It is ready for use immediately aer it is
powered ON.
500007, Rev P, Pg 16
500007, Rev P, Pg 17
7.6 GUIDANCE & MANUFACTURER’S DECLARATION
Electromagnec Compability (EMC)
The Skylife system is suitable for the electromagnec environment of typical homes, commercial
or hospital sengs.
During the immunity tesng described below the Skylife device connued to deliver treatment at a wavelength
of 430-475 nm at an intensity between 25 to 72 µW/cm2/nm.
WARNINGS:
• Portable RF communicaons equipment (including peripherals such as antenna cables and external antennas)
should be used no closer than 30 cm (12 inches) to any part of the Skylife device, including cables specied by
the manufacturer. Otherwise, degradaon of the performance of this equipment could result.
• The Skylife unit should not be used adjacent to or stacked with other equipment. If adjacent or stacked use
is necessary, the device should be observed to verify normal operaon. If operaon is not normal, the Skylife
device or the other equipment should be relocated.
• Use of accessories, transducers and cables other than those specied or provided by the manufacturer of this
equipment could result in increased electromagnec emissions or decreased electromagnec immunity of
this equipment and result in improper operaon.
Electromagnetic Emissions
Skylife™ is intended for use in the electromagnetic environment specified below. The customer or the user of Skylife™ should assure that it
is used in such an environment.
Emission Tests
Compliance
Electromagnetic Environment – Guidance
RF emissions CISPR 11
Group 1
Skylife™ uses RF energy only for its internal function. Therefore, its RF emissions are
very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11
Class B
Skylife™ is suitable for use in all establishments, including domestic establishments and
those directly connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions IEC 61000-3-2
Class B
Voltage fluctuations / flicker
emissions IEC 61000-3-3
Complies
Electromagnetic Immunity
Skylife™ is intended for use in the electromagnetic environment specified below. The customer or the user of Skylife™ should assure that it is
used in such an environment.
Immunity Test
Compliance Level
Electromagnetic Environment – Guidance
Electrostatic
discharge (ESD) IEC
61000-4-2
± 8kV contact
± 15kV air
The relative humidity should be at least 5 %
Electrical fast
transient / burst IEC
61000-4-4
± 2 kV for power supply
lines
Mains power quality should be that of a typical home commercial or hospital environment.
Surge IEC 61000-4-5
± 1 kV differential
mode
Mains power quality should be that of a typical home commercial or hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines IEC
61000-4-11
0% .5 Periods
0% 1 Period
70% 25 Periods
0% 5 sec
Mains power quality should be that of a typical home commercial or hospital environment.
If the user of Skylife™ requires continued operation during power mains interruptions, it is
recommended that Skylife™ is powered from an uninterruptible power supply.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
30A/m
Power frequency magnetic fields from common appliances in the home are not expected
to affect the device.
Power frequency magnetic fields should be at levels characteristic of a typical location in a
typical commercial or hospital environment. Keep Skylife™ away from sources of high
levels of power line magnetic fields (in excess of 30 A/m) to reduce the likelihood of
interference.”
NOTE: U
T
is the A/C. mains voltage prior to application of the test level.
Skylife™ is intended for use in the electromagnetic environment specified below. The customer or the user of the device should assure that
it is used in such an environment.
Immunity Test
IEC 60601 Test
Level
Compliance Level
Electromagnetic
Environment – Guidance
Conducted RF
IEC 61000-4-6
Radiated RF IEC
61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 Vrms
6 Vrms in ISM and
amateur radio bands
10 V/m
Skylife™ is suitable for the electromagnetic environment of typical
homes, commercial or hospital settings.
Class A

SKYLIFETM PHOTOTHERAPY SYSTEM
SkylifeTM is a trademark of Neolight LLC U.S.A.
This Skylife Phototherapy System is protected by US Patent ID: 15/143,277 and 15/882,485.
Other manuals for SKYLIFE
3
This manual suits for next models
1
Table of contents
Other NeoLight Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual