A(
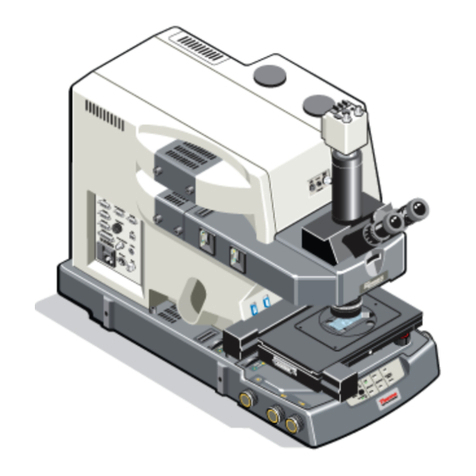
Adjusting the microscope for Koehler illumination.
1) Insert a sample and focus it using an objective that you typically use for imaging.
Tip: Focus on the edge of the coverslip to set the
course focus. Then focus up and down using fine
focus to locate sample.
2) Stop down (close) field aperture by rotating
dial on left side of microscope base. You may see a
round circle of light through the eyepieces that has
a blurry edge.
3) Adjust the condenser height (#1: black knob on left of condenser) until the circle of
light is smaller and the edges are sharp. This will appear as the octagon (8-sided) shape
of the aperture.
4) Open up the field aperture to fill ~half the area
with light.
5) Adjust the aperture centering with the two
silver knobs (#2 below the condenser turret). When
centered, open the field aperture until the entire field
is full of light.
Note: You may have to adjust the light for other
objectives. Lower magnification objectives have a
wider opening so will require opening the field
aperture more.
6) Close the condenser aperture by moving the
slider to the right. This is the position of maximum
diffraction. This will give additional contrast to the
sample but reduces resolution.
Open the condenser aperture until the light level does
not change. This is the position of maximum
resolution and minimum diffraction. Use this position
for all higher magnification objectives.
<Open……Close>
O | | | | | | | | | o
10 0
You have now aligned the microscope for routine brightfield imaging.