nobel biocare OsseoCare Pro User manual

OsseoCare™ Pro
Instructions for use - drilling unit
Manufactured
by

2
English
Disclaimer
Limitations of liability
This product is part of an overall concept and may only be used in conjunction with the associated original products according to the instructions and recommendation
of Nobel Biocare. Non-recommended use of products made by third parties in conjunction with Nobel Biocare products will void any warranty or other obligation,
express or implied, of Nobel Biocare. The user of Nobel Biocare products has the duty to determine whether or not any product is suitable for the particular patient and
circumstances. Nobel Biocare disclaims any liability, express or implied, and shall have no responsibility for any direct, indirect, punitive or other damages, arising out
of or in connection with any errors in professional judgment or practice in the use of Nobel Biocare products. The user is also obliged to study the latest developments
in regard to this Nobel Biocare product and its applications regularly. In cases of doubt, the user has to contact Nobel Biocare. Since the utilization of this product is
under the control of the user, they are his/her responsibility. Nobel Biocare does not assume any liability whatsoever for damage arising thereof. Please note that some
products detailed in this Instruction for Use may not be regulatory cleared, released or licensed for sale in all markets. Please note that not all products may have been
licensed in accordance with Canadian law.
For additional information on surgical procedures please consult the “Procedures & products” treatment guidelines available at nobelbiocare.com or request the latest
printed version from a Nobel Biocare representative.
Bien-Air Dental SA
Länggasse 60
Case postale
2500 Bienne 6, Switzerland
Tel. +41 (0)32 344 64 64
Fax +41 (0)32 344 64 91
Caution: The caution text “Federal (USA) law restricts the sale of this device to, or on the order of, a licensed physician or dentist” is shown on labels with
“Rx Only”
GMT 37341. Date of issue 13-11-2014. All rights reserved. Nobel Biocare, the Nobel Biocare logotype and all other trademarks used in this document are, if nothing else
is stated or is evident from the context in a certain case, trademarks of Nobel Biocare. iPad® and iCloud® are registered trademarks of Apple Inc. Product images in this
document are not necessarily to scale.
0120
3
English
Table of contents
Disclaimer 2
Table of contents 3
Description 4
Identication 4
Intended use 4
Indications 4
Contraindications 4
Compatibility 4
Limitation of liability 4
Hardware keys and elements 4
Explanation of symbols 5
Environment 6
Working 6
Transport and storage 6
Environmental protection and information for disposal 6
Technical description 7
Technical data 7
Electromagnetic compatibility 10
Precautions regarding Electromagnetic Compatibility (EMC) 10
Guidance & manufacturer’s declaration 10
Electromagnetic emission 10
Electromagnetic immunity 10
Recommended separation distances 11
Installation 12
Installing the OsseoCare™ Pro application on the iPad® 12
Installation of the OsseoCare™ Pro drilling unit 12
Installation of the iPad®on the OsseoCare™ Pro unit 13
On/off procedure 13
Operating the drilling unit 14
Blue button 14
Orange button 14
Grey button 14
Speed drive 14
List of errors and troubleshooting 15
Device operating error 15
Maintenance 16
Servicing 16
Information 16
Cleaning and disinfection 16
Important 16
General information and guarantee 16
Terms of guarantee 16

4
English
w e
t
y
u i o
q
a
w
r
e
rgf
d
s
Identication
Electronically controlled tabletop device for dentistry allowing the operation of an MX-i LED micromotor with variable speed control
by means of a pedal. A peristaltic pump conveys the physiological liquid via a disposable irrigation line without being contaminated.
It is essential to connect a supported iPad® to the device using the connector provided for this purpose.
TheimplantttingparametersaredenedbeforetheoperationusingtheOsseoCare™ProapplicationinstalledontheiPad®.
Intended use
The OsseoCare™ Pro system is intended for use in dental surgery, endodontics and implantology by dentists and surgeons in dental
ofcesandhospitalstocuthardandsofttissuewithappropriatetools.
TheOsseoCare™Prosystemisdesignedtocontroladentalmicromotorwhichcandriveadentalhandpiecettedwithappropriate
tools to cut hard and soft tissues in the mouth and to screw dental implants.
Indications
The OsseoCare™ Pro system does not specify a disease, condition or population and therefore the Indications for use are the same as
the Intended use.
Contraindications
Noneidentied.
Compatibility
The OsseoCare™ Pro drilling unit is compatible with iPad® devices iPad® 2, iPad® 3, iPad® 4, iPad® Air and iPad® Air 2. Please note that
no other iPad® devices can be used than the ones indicated here.
Limitation of liability
Bien-Air Dental shall not be held liable for any non-compliant use of the iPad®. The conditions for and restrictions on use set by Apple
mustberespected(jailbreak,hardwaremodication,etc.).
Description
Hardware keys and elements
q Adapter for iPad®
w Bracket support
e Pedal connector
r Peristaltic pump LID
t Micromotor connector
y Micromotor
u Irrigation ON/OFF control button on pedal
iCongurable‘Program’buttononpedal
o Button to reverse the rotation of the micromotor
on pedal
a Variable speed drive on pedal
s (100-240VAC) Mains connector
d Fuse holder
f Main switch
g Label

5
English
Explanation of symbols
CEMarkingwithnumberofthenotiedbody
0120
Protective earth (ground)
Fuse Ø 5 x 20 mm
Alternating current
Element sensitive to electrostatic discharges
Electrical security. Applied part type B.
CAUTION! Dangerous voltage.
WARNING!
Refer to the accompanying documents
Dangerofpinching.Donotputyourngersinrotatingparts.
Machine washable
Recyclable materials
Recyclable electrical and electronic materials
Sterilizableinautoclaveuptothespeciedtemperature.
135°C
Manufacturer (acc. 93/42/EEC article 1.2 (f))
Light
Use by date
Do not re-use
Sterilise with Ethylene Oxyde
Caution: Federal law (USA) restricts this device to sale by or on the order of a licensed healthcare practitioner.
Product containing phthalates
Main switch - Power OFF.
Main switch - Power ON.
DEHP
6
English
Environment
Working
Temperature: +10°C (50°F) to +25°C (77°F)
Relative humidity: 30% to 80%, including condensation
Atmospheric pressure: 700 hPa to 1060 hPa
Transport and storage
Environmental conditions for a period of maximum 15 weeks
Temperature: -25°C (-13°F) to +70°C (158°F)
Relative humidity: 10% to 100%, including condensation
Atmospheric pressure: 500 hPa to 1060 hPa
Environmental protection and information for disposal
Warning!
The device is not designed for use in an explosive atmosphere (anaesthetic gas).
The disposal and/or recycling of materials must be performed in accordance with the legislation in force.
This device and its accessories must be recycled.
Electrical and electronic equipment may contain dangerous substances which constitute health and environmental hazards.
The user must return the device to its dealer or establish direct contact with an approved body for treatment and recovery of
this type of equipment (European Directive 2002/96/EC).
7
English
Technical description
Technical data
Voltage
100 – 240 VAC, 50 / 60 Hz
Fuses
2 fuses T4.0A L 250 VAC, breaking capacity 40A
Power demand
– 100 V / 300 VA
– 240 V / 300 VA
Classication
Class IIa in accordance with European Directive 93/42/EEC concerning medical devices.
Electric insulation class
Class I, per IEC 60601-1
(apparatus protected against electric shocks).
Degree of protection
IP 40 (protection against insertion of objects larger than 1 mm).
Dimensions L x W x H
242 x 244 x 102 mm.
Height with bracket 482 mm
Weight
Housing 2.8 kg Pedal 830 g
Cable 105 g Bracket 115 g
Languages
English
List of errors & troubleshooting
Turn to page 15.
Bracket for physiological liquid ask
Stainless steel
Intended for use with
MX-i LED micromotor REF 2100264
Cable for micromotor REF 2100163
Contra-angle CA 20:1 L Micro-Series, light REF 2100263
Contra-angle CA 20:1 L KM Micro-Series, light REF 2100263
Warning!
The use of the system with other handpieces, motors or cables has not been validated / certied.

8
English
Peristaltic pump
Pump delivery: From 30 to 150 ml/min. (5 levels)
Hose for pump: External Ø 5.60 mm
Internal Ø 2.40 mm
Wall thickness 1.60 mm
Foot control
REF 1600631-001
Dimensions (LxWxH) 250 x 205 x 54 mm
With handle: 250 x 205 x 144 mm
The pedal is waterproof (IP X8 in accordance with CEI 529).
Cables
Length of cables:
Pedal cable 2.90 m
Motor cable 2.00 m
Parts applied (per IEC 60601-1)
MX-i LED micromotor REF 1600875-001
Cable for MX-i LED micromotor REF 1600606-001
CA 20:1 L Micro-Series REF 1600873-001
CA 20:1 L KM Micro-Series REF 1600874-001
Irrigation lines REF 1500984-010
KM Irrigation lines REF 1501635-010
Warning!
– To prevent any risk of electric shock, this device must be connected only to a power supply network provided
with protective earth.
– Modication of the device is forbidden.
– The system is not adapted for use in the presence of inammable gases (e.g. anesthetic gas).
– Do not attempt to open the apparatus when it is connected to the electric mains. Beware of electric shocks.

9
English
Set OsseoCare™Pro CA 20:1 L MS REF 1700470-001
1 x REF 1303393-0011 x REF 1600870-001 1 x REF 1306205-001
CA 20:1 L
Micro-Series
1 x REF 1600873-001
1 x REF 1600875-001 1 x REF 1305949-001 1 x REF 1301575-001 1 x REF 1501746-010
10 x
1 x REF 1500984-010
1 x REF 1600631-001
1 x REF 1306026-001
1 x REF 1306025-001
1 x REF 1300065-001
1 x REF 1300066-001
1 x REF 1300067-001
10 x
1 x REF 1303711-010 1 x REF 1305947-001 1 x REF 1600606-001
Set OsseoCare™ Pro CA 20:1 L MS KM REF 1700471-001
1 x REF 1305949-001 1 x REF 1301575-001 1 x REF 1501746-010
CA 20:1 L KM
Micro-Series
1 x REF 1600874-001 1 x REF 1303393-001
1 x REF 1600870-001 1 x REF 1306205-001 1 x REF 1600875-001
10 x
1 x REF 1501635-010
1 x REF 1600631-001
10 x
1 x REF 1501621-010
10 x
1 x REF 1303711-010 1 x REF 1305947-001
1 x REF 1306026-001
1 x REF 1306025-001
1 x REF 1300065-001
1 x REF 1300066-001
1 x REF 1300067-001
1 x REF 1600606-001
Optional
CA 20:1 L
Micro-Series
1 x REF 1600873-001
CA 20:1 L KM
Micro-Series
1 x REF 1600874-001 1 x REF 1600606-001
1 x REF 1600875-001 1 x REF 1303393-0011 x REF 1305947-001 1 x REF 1501746-010 1 x REF 1600631-001
1 x REF 1301575-001
10 x
1 x REF 1501621-010
10 x
1 x REF 1500984-010
10 x
1 x REF 1501635-010
10 x
1 x REF 1301560-010
10 x
1 x REF 1303711-010
10
English
Electromagnetic compatibility
Precautions regarding Electromagnetic Compatibility (EMC)
Electro-medical equipment needs special precautions regarding EMC and needs to be installed and put into service according to the EMC
information provided in this document.
OsseoCare™Pro complies with the EMC requirements according to IEC 60601-1-2. Radio transmitting equipment, cellular phones, etc.
shall not be used in close proximity to the device since they could inuence the performance of the device. Particular precaution is
required when using strong emission sources such as High Frequency surgical equipment and similar equipment so that the HF cables
are not routed on or near the device. If in doubt, please contact your local service and repair center (see the contacting details on www.
nobelbiocare.com/osseocare).
OsseoCare™Pro should not be used adjacent to, or stacked with, other equipment. If adjacent or stacked use is necessary, OsseoCare™Pro
shouldbemonitoredtoverifynormaloperationinthecongurationinwhichitwillbeused.
Guidance & manufacturer’s declaration
Electromagnetic emission
OsseoCare™Proisintendedforuseintheelectromagneticenvironmentspeciedbelow.
The customer or the user of OsseoCare™Pro should ensure that it is used in such an environment.
Electromagnetic immunity
OsseoCare™Proisintendedforuseintheelectromagneticenvironmentspeciedbelow.
The customer or the user of OsseoCare™Pro should ensure that it is used in such an environment.
Warning!
The use of accessories, transducers and ca bles other than those specied, with the exception of transducers and
cables sold by Bien-Air Dental as replacements parts for internal components, may result in increased emissions
or decreased immunity of OsseoCare™Pro.
Dental professionals need to be aware of potential electromagnetic interference between electronic dental devices
and active implantable medical devices, and should always inquire about any devices implanted in the patient.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1 OsseoCare™Pro uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class B
OsseoCare™Pro is suitable for use in all establishments, including domestic establishments
and those directly connected to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Compliant
Voltageuctuations/ickeremissions
IEC 61000-3-3
Not applicable
Immunity test IEC 60601 test level Compliance Electromagnetic environment - guidance
Electrostatic discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floorsshouldbewood,concreteorceramictile.Ifoors
are covered with synthetic material, the relative humidity
should be at least 30%.
Electrical fast transient burst
IEC 61000-4 -4
±2 kV for power supply lines
±1 kV for lines no input/output
±2 kV for power
supply lines
±1 kV for lines no
input/output
Mains power quality should be that of a typical
commercial or hospital environment.
Shock waves
IEC 61000-4 -5
±0.5 kV line to line
±1 kV line to line
±0.5 kV line to earth
±1 kV line to earth
±2 kV line to earth
±0.5 kV line to line
±1 kV line to line
±0.5 kV line to earth
±1 kV line to earth
±2 kV line to earth
Mains power quality should be that of a typical
commercial or hospital environment.
Voltage dips and outages
IEC 61000-4-11
<5% UT (>95% dip in UT) for 0.5 cycle
40% UT(60% dip in UT) for 5 cycles
70% UT(30% dip in UT) for 25 cycles
<5% UT(>95% dip in UT) for 5 sec
<5% UT(>95% dip in UT)
for 0.5 cycle
40% UT(60% dip in UT)
for 5 cycles
70% UT(30% dip in UT)
for 25 cycles
<5% UT(>95% dip in UT)
for 5 sec
Mains power quality should be that of a typical
commercial or hospital environment. If the user of
OsseoCare™Pro requires continued operation during
power mains interruptions, it is recommended that
OsseoCare™Pro be powered from an uninterruptible
power supply or a battery.
Power frequency (50 Hz)
magneticeld
IEC 61000-4-8
3 A/m 3 A/m
Powerfrequencymagneticeldsshouldbeatlevels
characteristic of a typical location in a typical commercial
or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
Essential performance: The essential performance is the maintaining of the visual lighting intensity of the LED and the maintaining of motor speed. Maximum
allowed speed deviation is ± 5%.
11
English
OsseoCare™Proisintendedforuseintheelectromagneticenvironmentspeciedbelow.
The customer or the user of OsseoCare™Pro should ensure that it is used in such an environment.
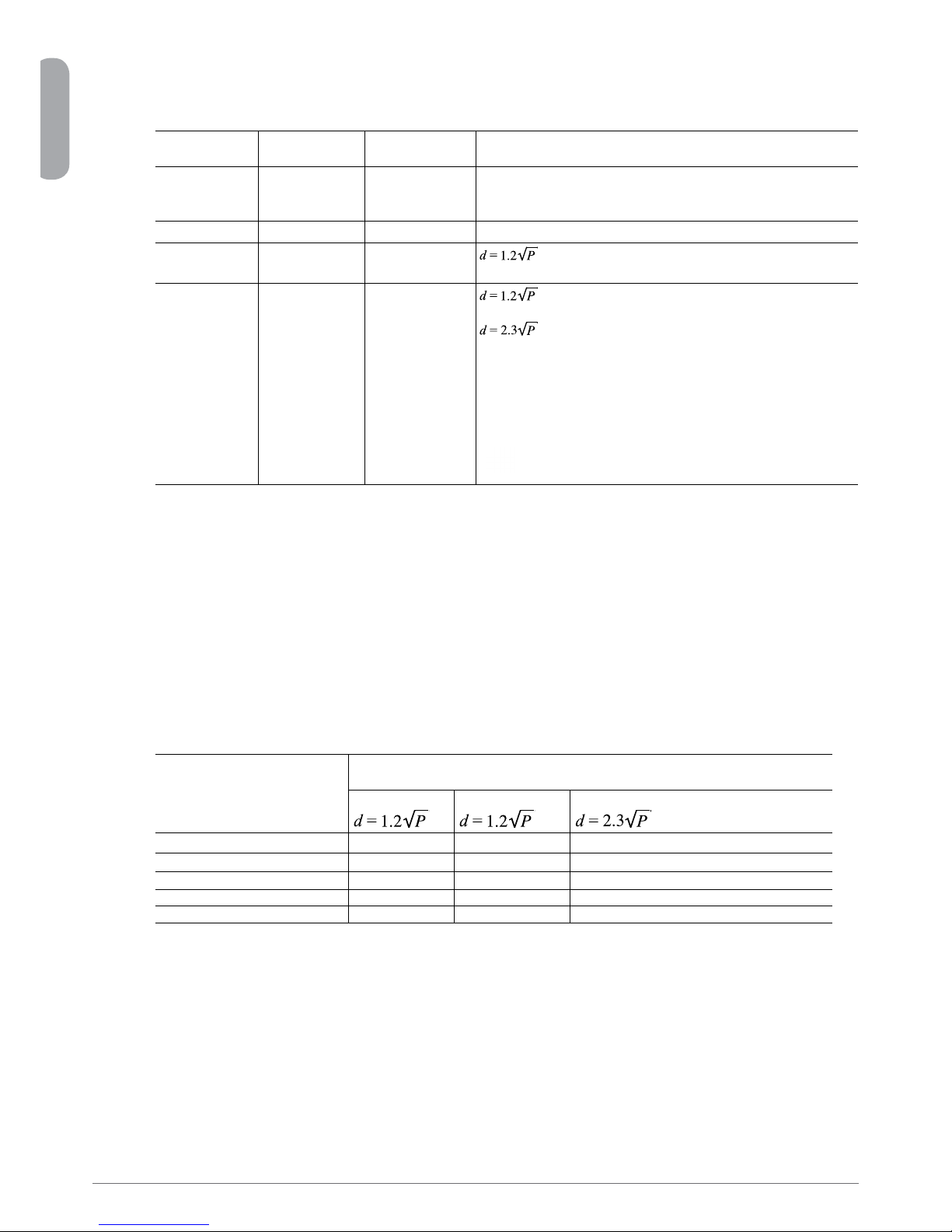
Recommended separation distances
(between portable and mobile RF communications equipment and the OsseoCare™ Pro)
The OsseoCare™Pro is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer
or the user of the OsseoCare™Pro can help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the OsseoCare™Pro as recommended below, according to the maximum output
power of the communications equipment.
Immunity test IEC 60601 test
level
Compliance
level
Electromagnetic environment - guidance
Portable and mobile RF communications should be used no closer to any part of
OsseoCare™ Pro, including cables, than the recommended separation distance
calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
3V
radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2,5 GHz
3 V/m 80 MHz to 800 MHz
800 MHZ to 2.5 GHz
Where
P
is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and
d
is the recommended separation
distance in metres (m)
FieldstrengthsfromxedRFtransmitters,asdeterminedbyanelectromagnetic
site survey (a), should be less than the compliance level in each frequency range
(b). Interference may occur in the vicinity of equipment marked with this symbol.
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE2Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationisaffectedbyabsorptionandreectionfromstructures,objectsandpeople.
(a)Fieldstrengthsfromxedtransmitters,suchasbasestationsforradio(cellular/cordless)telephonesandlandmobileradios,amateurradio,AMandFM
radiobroadcastandTVbroadcastcannotbepredictedtheoreticallywithaccuracy.ToassesstheelectromagneticenvironmentduetoxedRFtransmitters,an
electromagneticsitesurveyshouldbeconsidered.IfthemeasuredeldstrengthinthelocationinwhichtheOsseoCare™ProisusedexceedstheapplicableRF
compliance level above, the OsseoCare™ Pro should be observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as reorienting or relocating the OsseoCare™ Pro.
(b)Overthefrequencyrange150kHzto80MHz,eldstrengthsshouldbelessthan3V/m.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 38 38 73
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in metres (m) can be estimated using the equation
applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE2Theseguidelinesmaynotapplyinallsituations.Electromagneticpropagationisaffectedbyabsorptionandreectionfromstructures,objectsandpeople.

12
English
Installing the OsseoCare™ Pro application on the iPad®
q Open the App StoreSM by tapping on the App StoreSM icon on the iPad®.
w Find the OsseoCare™ Pro application using the search window at the top right-hand side of the screen.
e Tapon‘Free’.
r Thentapon‘Install’toinstalltheOsseoCare™Proapplication.
Caution!
The iPad® must be connected to the Internet correctly before the App Store is opened. Refer to Apple’s instructions for
directions regarding appropriate use of the iPad®.
Installation of the OsseoCare™ Pro drilling unit
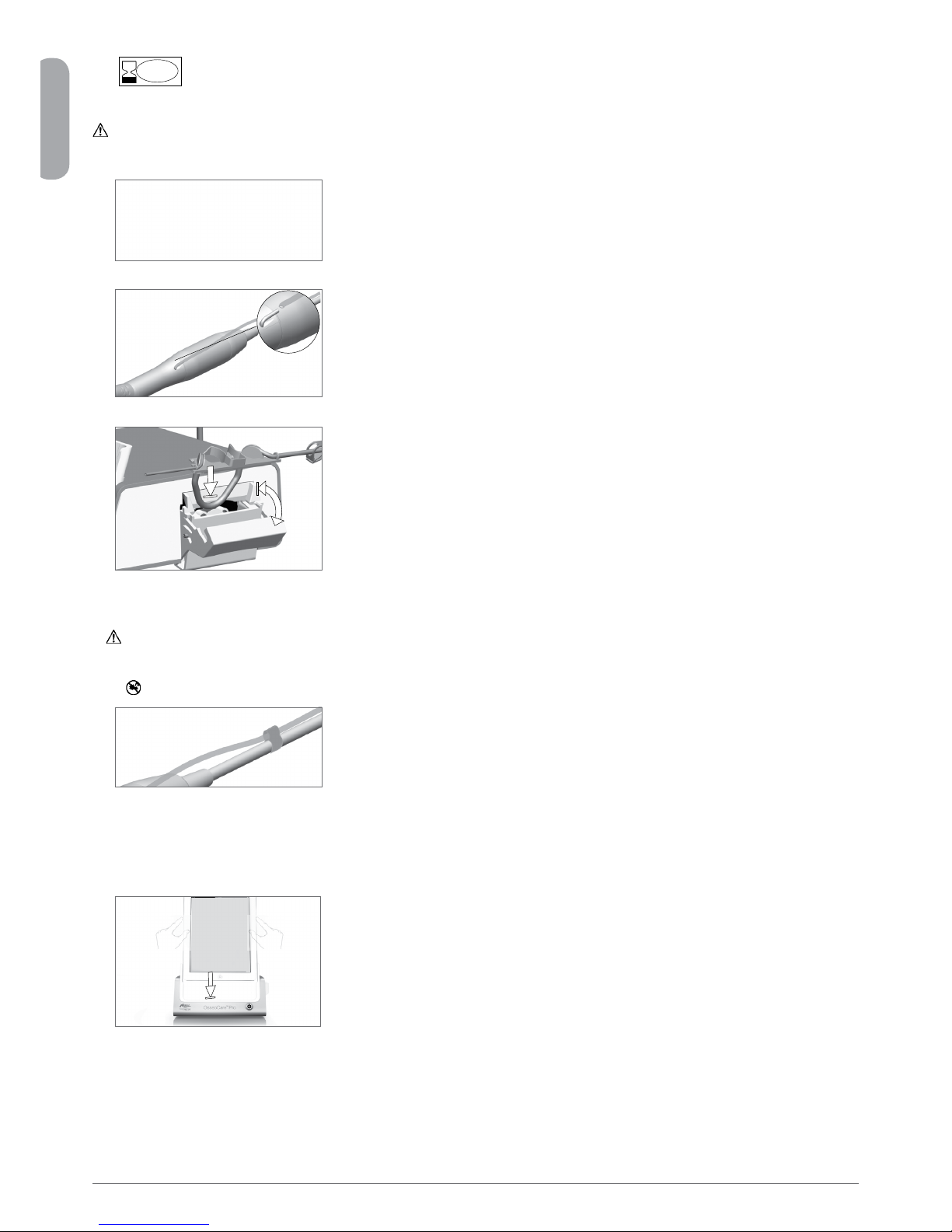
q If necessary, use the screwdriver to change the adapter for the iPad® model being used. Please ensure that, during this action, the
OsseoCare™ Pro drilling unit is not connected to the electrical outlet.
w OsseoCare™Promaybepositionedonatable,onatrolleyoranothersurface,butinnocircumstancesontheoor.Thepowerplug
is the device for disconnection in case of problems, and it must be easily accessible at all times.
e The fuse box may be opened with a screwdriver. 100 - 240 Vac = fuse T-4.0 A L 250 VAC (REF 1301560-010)
r The equipment is powered by your line voltage (100 - 240 Vac). Connect the power cable to the plug.
t Connect the pedal cable to the output provided on the rear panel, guiding the connector and plug by means of the index pin on the
connector.
Warning!
Do not raise the pedal using the connection cable.
y Connect the micromotor cable to the motor output, guiding the connector and plug by means of the index pin on the connector.
u Alignandattachthebrackettothehousingprovidedontheconsole’srearandsuspendtheaskorbottle.
Installation
ON/OFF
r
e
t
u
y
13
English
i Check the packaging integrity, as well as the expiry date of the irrigation line on the label.
Warning!
Only lines supplied by Bien-Air Dental ensure trouble-free operation. These lines are sterile and for single use. Reuse may
result in microbiological contamination of the patient.
o Remove the single-use sterile irrigation line from its pouch.
a Connecttheexiblehoseoftheirrigationlinetothespraytubeofthehandpieceorcontra-angle.
s Install the plastic cassette in the peristaltic pump. Check that the cassette is clipped correctly. Close the pump lid. If there is any
resistance when closing, open the lid again and check the correct positioning of the cassette.
Caution!
Do not run the pump when the lid is open.
Danger of pinching
d Perforatethecapofthephysiologicalliquidaskwiththepointedendoftheirrigationlineafterremovingtheprotectivecap.Attach
the irrigation line onto the motor cable using the attachment collars (REF 1303711-010).
Installation of the iPad®on the OsseoCare™ Pro unit
q Remove the single-use sterile protection sheet for iPad® from its pouch and stick it on the screen. Refer to the instructions on the
back of the pouch.
w Connect the iPad® to the OsseoCare™ Pro by sliding it carefully along the adapter. Drape the back of the iPad® with appropriate
material (e.g. iPad® protective film) to prevent contamination.
On/off procedure
The device can be switched on and off safely using the main switch on the iPad® and OsseoCare™ Pro.
YYYY/MM i
o
a
s
REF 1303711-010
d
w

14
English
Operating the drilling unit
When the iPad® is installed on the OsseoCare™ Pro and everything is set up and connected correctly, the drilling unit can be operated.
The OsseoCare™ Pro device is equipped with an ergonomic foot pedal with three colored buttons. These give you full control over speed,
rotation direction and drill selection. They also allow you to switch irrigation on and off easily, or to jump between drilling and implant
insertion without touching the iPad® screen.
Blue button
Thebluebuttonisthecontrolbuttontoswitchbetweentheirrigationlevelstatuses‘On’and‘Off’.Whentappingthebluebuttonabeeping
sound is made:
— A single beep means irrigation is switched ON.
— A double beep means irrigation is switched OFF.
Orange button
A short press on the orange button takes you one step further when moving between different values in the quick select bar or drills in a
drill set.
When you press and hold down the orange button, the app switches between the drilling page and the implant insertion page. Another
press and hold switches back to the other page. Three short beeps (two low and one higher beep) can be heard whenever you switch
between these two pages by means of the orange button.
Grey button
Press the grey button to reverse the rotation of the micromotor. A beeping sound is made to indicate the rotation direction.
— A single beep means the drill is rotating forward.
— A double beep means it is rotating in reverse.
— When pressing down the foot pedal, the Drill Direction indicator in the application will blink.
Speed drive
The large central pedal button is the variable speed drive on the device for both drilling speed and implant insertion speed.

15
English
List of errors and troubleshooting
ERROR 1
Pedal connection is
missing
The pedal is not connected! Please
check the pedal connection.
The pedal is not properly connected. 1. Check pedal connection.
2. If the problem persists, contact Bien-Air Dental SA.
ERROR 3
Irrigation pump general
failure
Irrigation pump fault! Please contact
Bien-Air Dental SA.
Irrigation pump electrical failure.
Irrigation pump motor overheats.
Contact Bien-Air Dental SA.
ERROR 4
Motor connection is
missing.
The motor is not connected! Please
check the motor connection.
Motor phase missing failure. Motor is
not properly connected.
1. Check motor connection.
2. If the problem persists, contact Bien-Air Dental SA.
ERROR 5
Motor cable failure Motor cable failure. Motor drive power protection failure.
The motor cable may be defective.
1. Replace motor cable.
2. If the problem persists, contact Bien-Air Dental SA.
ERROR 6
Motor drive
overtemperature
Overall system overheating!
Please wait until cool.
Motor drive overtemperature failure. 1. Wait for the system to cool down.
2. If the problem persists, contact Bien-Air Dental SA.
GEN ERROR [FailCode]
GEN ERROR [FailCode]
System electrical failure
Electrical system fault!
Bien-Air Dental SA.
[FailCode] = EC100: Motor drive
communication failure
[FailCode] = EC101: Motor drive under
voltage failure
[FailCode] = EC102: Motor drive over
voltage failure
[FailCode] = EC120: Motor drive other
failure
1. Switch OFF the unit.
2. Disconnect the iPad® device from the drilling unit.
3. Close the OsseoCare™ Pro app.
4. Switch the drilling unit back ON.
5. Reconnect the iPad® device to the drilling unit.
6. Restart the OsseoCare™ Pro app.
7. If the problem persists, contact Bien-Air Dental SA.
Message Cause of error Action
Release the pedal The pedal is pressed when starting
the device.
The motor is blocked for more than
2 sec.
Safety Release the pedal and press again.
The motor control card limits the
power supplied to the motor to
prevent motor overheating.
Safety Avoid extended use
Device operating error
The following errors may occur during operation of the device
16
English
Maintenance
Only use original Bien-Air Dental maintenance products and parts or those recommended by Bien-Air Dental. Using other products or parts
may cause operational failure and/or void the guarantee.
Servicing
Neverdisassemblethedevice.Foranymodicationandrepair,werecommendthatyoucontactyourregularsupplierorBien-AirDental
directly. Bien-Air Dental asks the user to have its dynamic instruments checked or inspected at least once a year.
Information
Thetechnicalspecications,illustrationsanddimensionscontainedintheseinstructionsaregivenonlyasaguide.Theymaynotbethe
subject of any claim.
For all additional information, please contact Bien-Air Dental SA at the address indicated on the back cover.
Cleaning and disinfection
— Disinfect the surfaces of the console by rubbing with a clean cloth soaked in a suitable disinfectant (e.g. isopropyl alcohol) for
about 15 seconds.
— Do not immerse in disinfectant solution.
— Not designed for an ultrasonic bath.
— Use a new sterile irrigation line for each patient.
— Use a new sterile protective sheet for each patient.
Important
For maintenance of: see instructions
MX-i LED micromotor REF 2100264
Cable for micromotor REF 2100163
Contra-angle CA 20:1 L Micro-Series REF 2100263
Contra-angle CA 20:1 L KM Micro-Series REF 2100263
General information and guarantee
Thedevicemustbeusedbyqualiedprofessionalsincompliancewiththecurrentlegalprovisionsconcerningworkplacesafety,healthand
accident prevention measures, and these working instructions. In accordance with such requirements, the operators:
— must only use devices that are in perfect working order; in the event of irregular functioning, excessive vibration, abnormal heating
or other signs that may indicate malfunction of the device, the work must be stopped immediately; in this case, contact a repair
center that is approved by Bien-Air Dental.
— must ensure that the device is used only for the purpose for which it is intended, must protect themselves, their patients and third
parties from any danger, and must avoid contamination through the use of the product.
Terms of guarantee
Bien-Air Dental grants the user a guarantee covering all functional defects, material or production faults.
The device is covered by this guarantee for 24 months from the date of invoicing.
Incaseofajustiedclaim,Bien-AirDentaloritsauthorisedrepresentativewillfullthecompany’sobligationsunderthisguaranteeby
repairing or replacing the product free of charge. Any other claims, of whatever nature, in particular in the form of a claim for damages and
interest, are excluded.
Bien-Air Dental shall not be held responsible for damage or injury and the consequences thereof, resulting from:
— excessive wear and tear
— improper use
— non-observance of the instructions for installation, operation and maintenance
— unusualchemical,electricalorelectrolyticinuences
— poor connections, whether of the air, water or electricity supply.
Theguaranteedoesnotcoverexible“breoptic”typelightconductors,oranypartsmadeofsyntheticmaterials.
The guarantee shall become null and void if the damage and its consequences are due to improper manipulation of the product, or
modicationstotheproductcarriedoutbypersonsnotauthorisedbyBien-AirDental.
Claims under the terms of the guarantee will be considered only on presentation, together with the product, of the invoice or the consignment
note, on which the date of purchase, the product reference and the Serial No. should be clearly indicated.
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











