• Use the device only in dust-free conditions, otherwise treatment could be compromised.
• Do not wash the device under running water or by immersion and keep it safe from being
sprayed by water or other liquids.
• Do not expose the device to particularly extreme temperatures.
• Do not place the device near sources of heat, in direct sunlight or in excessively hot
rooms.
• Do not obstruct or put objects into the lter or its related housing in the device.
• Never obstruct the air vents located on both sides of the device.
• Always use it on a rigid surface that is clear of obstacles.
• Make sure there is no material obstructing the air vents before each use.
• Do not put any objects in the air vents.
• Repairs, including the replacement of the supply cord, are to be carried out by Nuvita
authorised personnel only, by complying with the information provided by the
manufacturer.
• The average expected duration for the compressor is: 400 hours.
• WARNING: Do not modify this device without authorisation from the manufacturer.
• The Manufacturer, the Vendor and the Importer shall be held responsible for safety,
reliability and performance only if: a) the device is used in compliance with the
instructions for use b) the wiring where the device is being used is in compliance with
safety regulations and current laws.
• Interactions: the materials used in contact with medication have been tested with a
vast range of medications. However, in view of the variety and continuous evolution of
pharmaceuticals, interactions cannot be ruled out. We recommend using the medication
as soon as possible once it has been opened and preventing prolonged exposure in the
nebuliser cup.
• The manufacturer must be contacted about any problems and/or unexpected events
concerning operation and for any clarications on use, maintenance/cleaning.
• Interactions: The materials used in the medical device are biocompatible in accordance
with the provisions of Directive 93/42 EC and subsequent amendments. However, the
possibility of occurrence of allergic reactions cannot be entirely excluded.
OPERATING INSTRUCTIONS
Before each use, clean hands thoroughly and clean the device as described in the
section on“CLEANING, SANITIZATION AND DISINFECTION”. During use, it is advisable
to protect yourself from any dripping. It is recommended that each person use their
own nebulizer cup and accessories to prevent risk of infection due to contamination.
This device is suitable for the administration of medical substances and not, for
which the administration via aerosol is foreseen; these substances are to be in any
case prescribed by the Doctor. In case of too thick substances, the dilution with a suit-
able physiological solution could be needed, according to the medical prescription.
1. Plug the power cord (A6) into a power socket corresponding to the voltage of the unit.
This must be positioned so that it is not dicult to make the disconnection from the
mains.
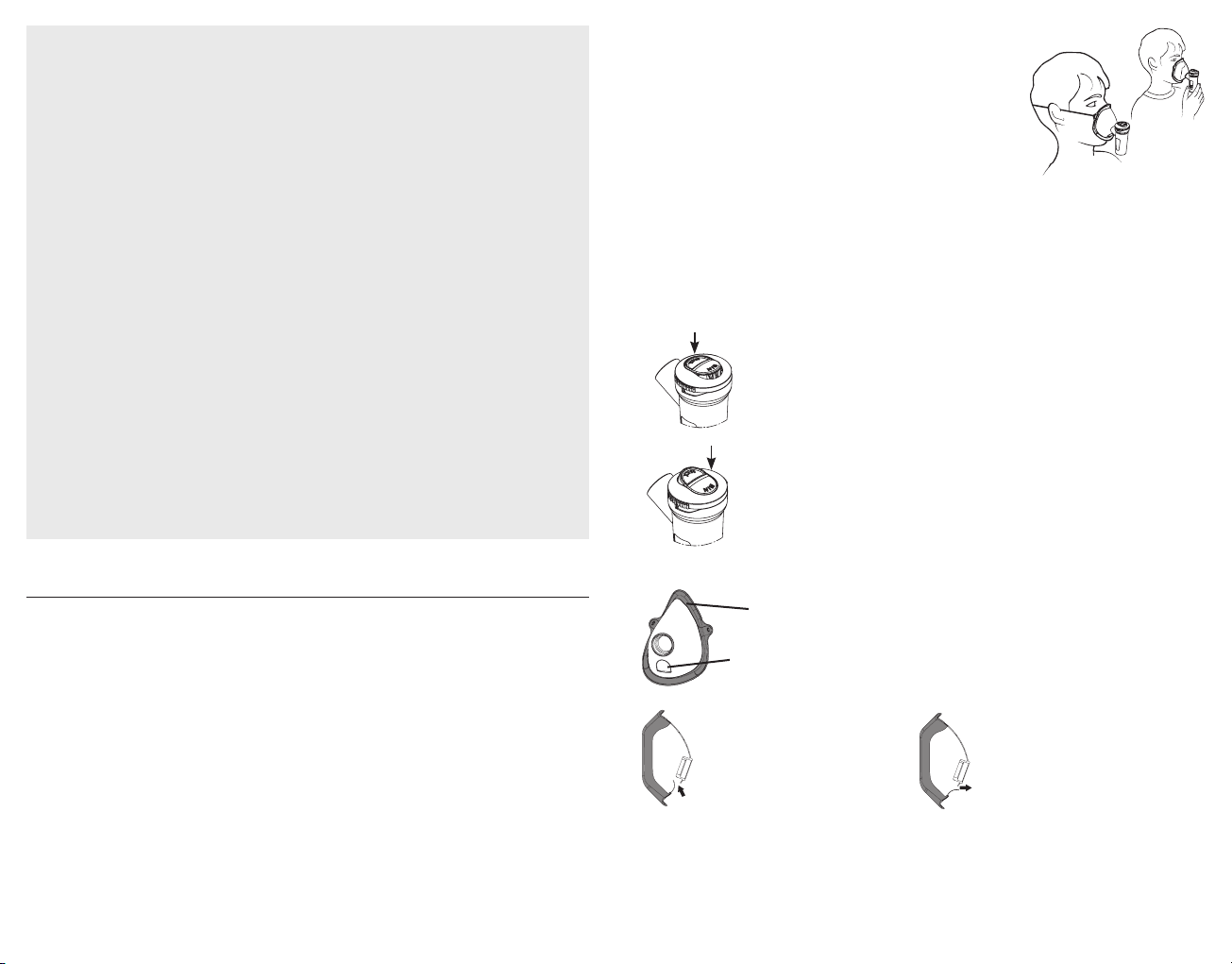
2. Insert the nozzle (C1.2) in the upper part (C1.3) pressing as shown by the 2 arrows in the
“Connection diagram”in point C1. Insert the Speed selector (C1.4) in the upper part (C1.3)
as shown in the “Connection diagram” in point C1. Pour the medication prescribed by
the doctor into the lower part (C1.1). Close the nebuliser by turning the upper part (C1.3)
clockwise.
3. Connect accessories as indicated in the“Connection diagram”on the cover.
4. Sit comfortably holding the nebuliser in your hand, place the mouthpiece onto your
mouth or alternatively use the nose piece or mask. Should
you opt for the mask accessory, place it on your face as
shown in the picture (with or without using the elasticated
strap).
5. Turn on the device using the switch (A1) and then breathe
in and exhale deeply.
6. When the treatment is nished, shut o the device and
unplug the power cord.
WARNING: If after the therapy session moisture accumulates
in the tube (B), remove the tube from the nebulizer and
dry it using the device’s compressor fan; this operation will prevent mold from forming
inside the tube.
SoftTouch masks have an outer edge made of soft
biocompatible material that ensures excellent ad-
herence to the face, and is also equipped with an
innovative Dispersion Limiting Device. These dis-
tinctive elements that distinguish it allow greater
sedimentation of medication in the patient and also
limit dispersion.
During the inspiratory phase, the
tab, acting as a Dispersion Lim-
iting Device, bends inwards to-
wards the mask.
During the expiratory phase, the
tab, acting as a Dispersion Limit-
ing Device, bends outwards from
the mask.
HOW TO USE THE“RF7 DUAL SPEED”NEBULIZER WITH THE SPEED SELECTOR
Professional and fast, this device is suitable for administering all types of medications,
including more expensive ones,even in patients with chronic diseases. The geometry
of the internal lines of the RF7 Dual Speed nebulizer ensures the ideal granulometry for
active treatment all the way down to the lower respiratory tract.
To speed up the inhalation therapy, move the speed selector button
(C1.4) by pressing on MAX with your nger.
To increase the eectiveness of the inhalation therapy, press with your
nger on the wording MIN of the speed selector (C1.4). In this position,
the speed selector acts as a valve and allows nebulizing the optimal
amount of drug to the lower respiratory tract, reducing its dispersion in
the environment.
SOFTTOUCH FACE MASKS
Soft
biocompatible
material
Dispersion
Limiting Vent