Spinal-Stim®Instruction Manual
Table of Contents
Prescription Information .............................................................................
• Indications............................................................................................
• Contraindication...................................................................................
• Warnings ..............................................................................................
• Precautions...........................................................................................
• Adverse Events .....................................................................................
Device Information......................................................................................
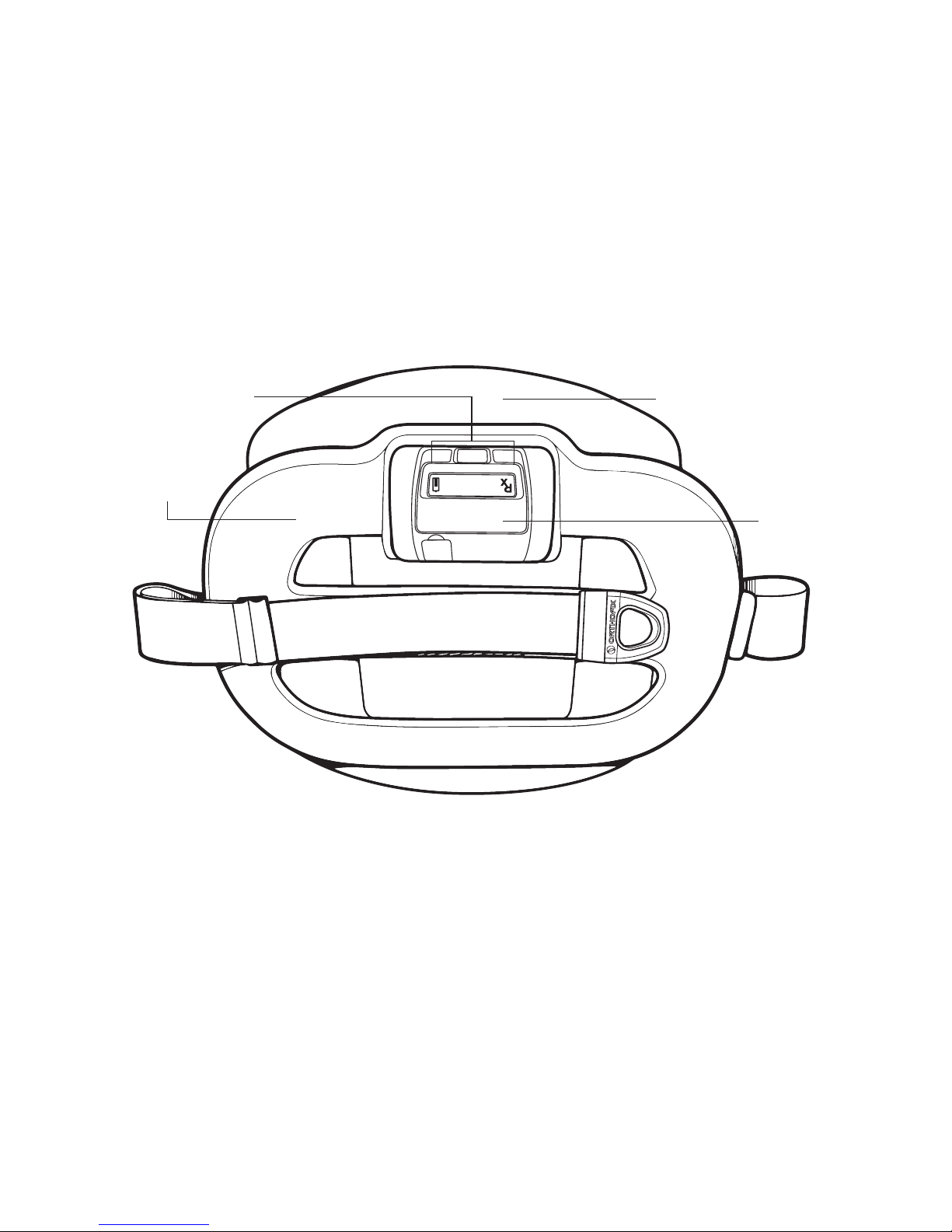
• Device Description................................................................................
• Device Life ............................................................................................
Device Operation.........................................................................................
• Turning the Device On and Off.............................................................
• Treatment Instructions .........................................................................
• Timing of Treatment Sessions...............................................................
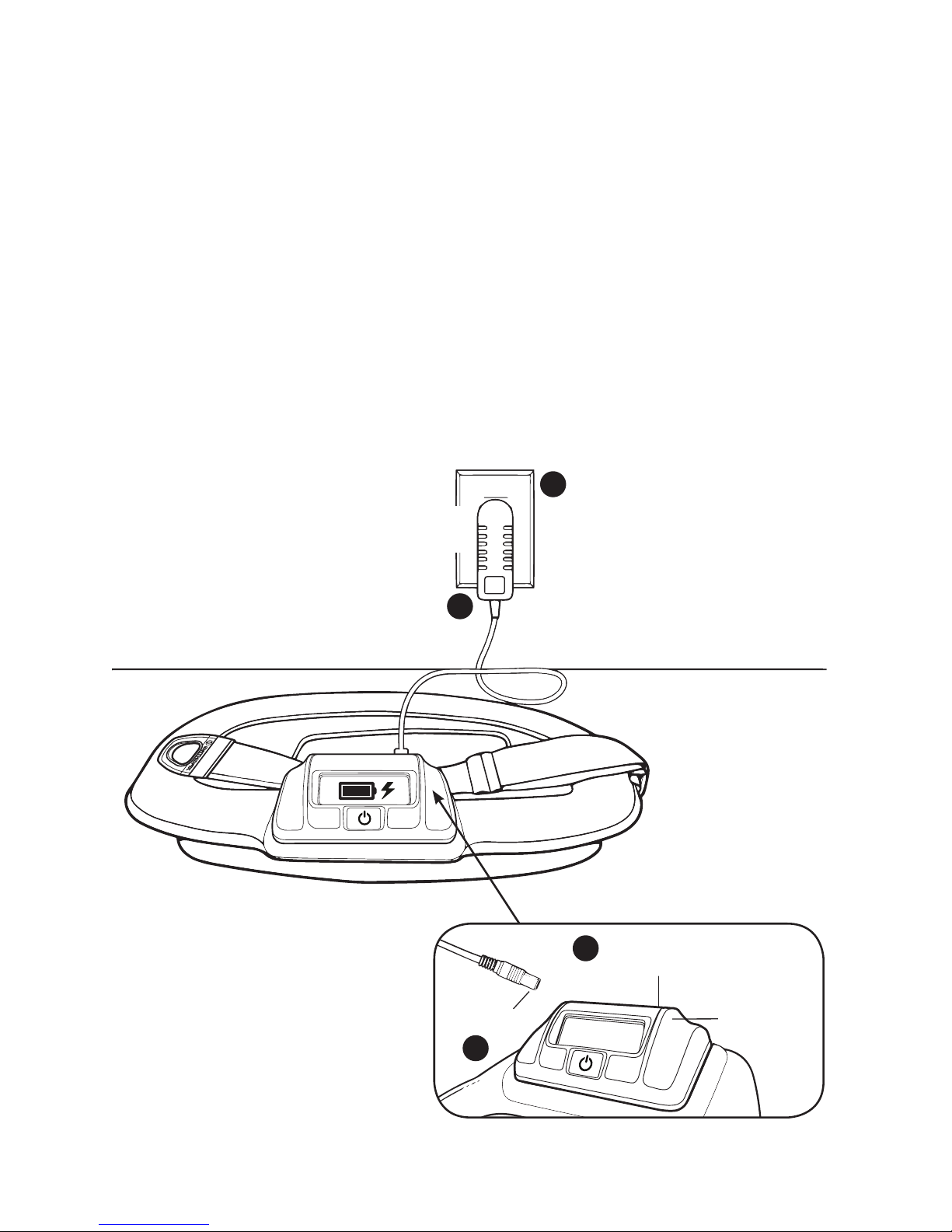
• Charging the Battery ............................................................................
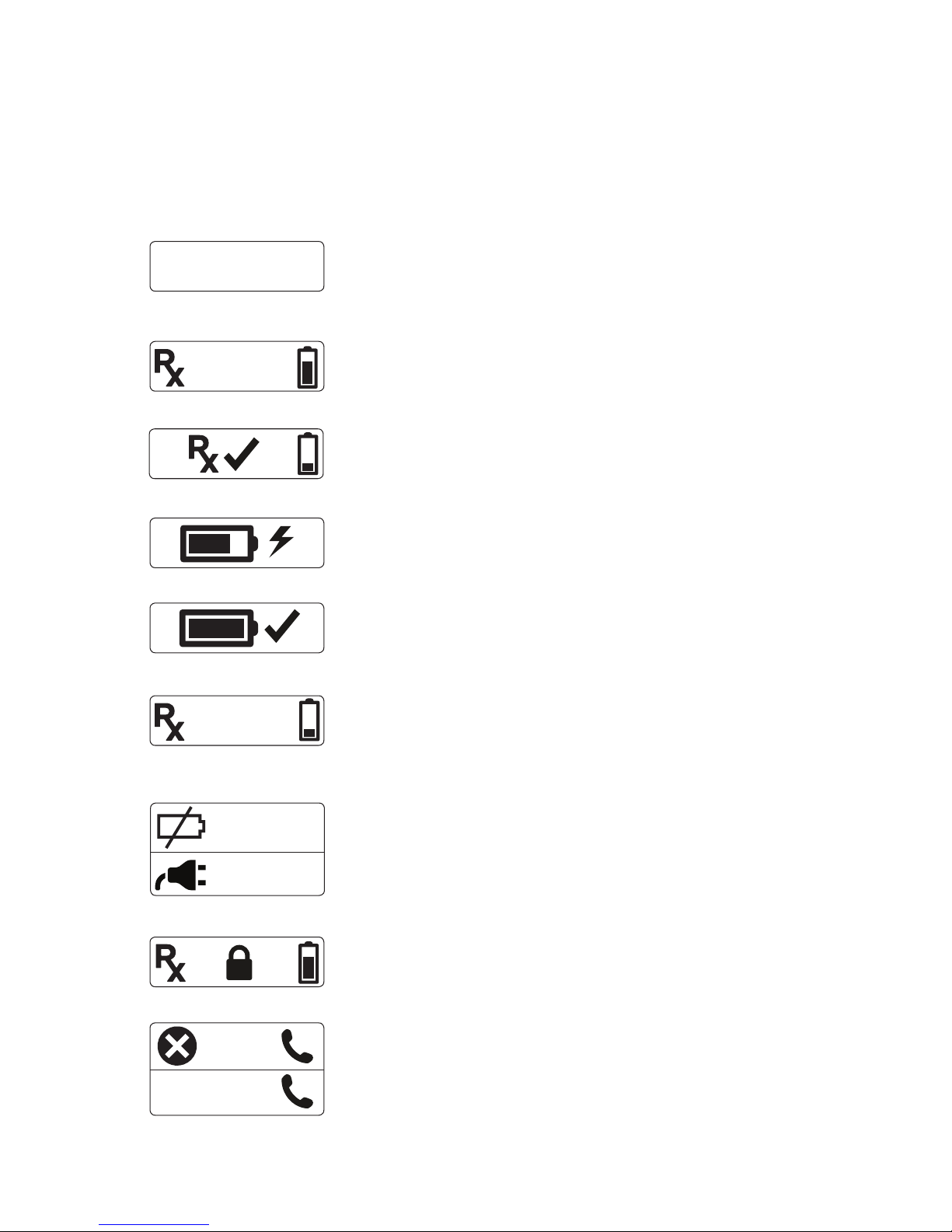
• Visual and Audio Indicators..................................................................
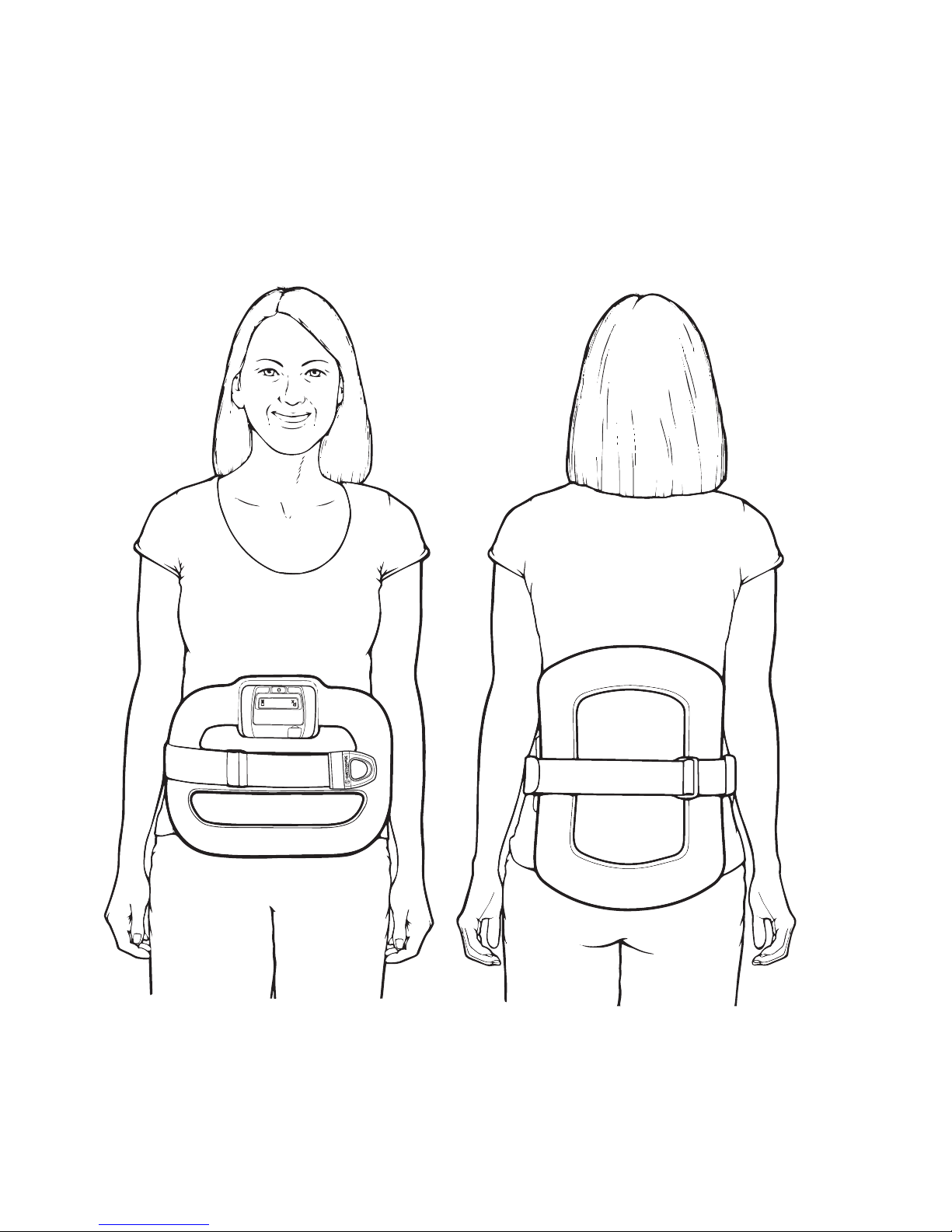
• Wearing the Device ..............................................................................
• Sizing the Device ..................................................................................
Device Accessories.....................................................................................
Device Use and Care .................................................................................
• Care and Cleaning ..............................................................................
• Storage...............................................................................................
• Travel..................................................................................................
• Disposal..............................................................................................
• Service ................................................................................................
Clinical Information...................................................................................
• Clinical Data Summary .......................................................................
• Adjunct Clinical Trial...........................................................................
• Failed Fusion Clinical Trial...................................................................
Equipment Classification...........................................................................
Compliance Statements ............................................................................
Warranty ...................................................................................................
Device Box Components
1 – Spinal-Stim
1 – Power Supply
1 – Literature Pack
Orthofix Patient Services: 800-535-4492 or 214-937-2718
To learn more about Orthofix, please visit our website at www.orthofix.com.
1
1
1
1
1
1
2
2
2
3
3
4
4
4
6
7
9
11
11
11
11
12
12
12
13
13
13
14
16
17
19