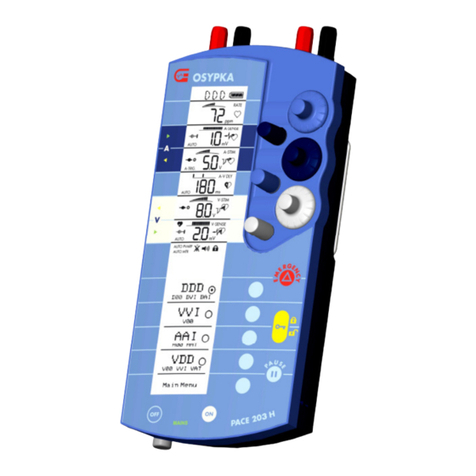
PACE 203H – Instructions for Use
5I-17-016X-B-20 9 / 139
1 Preface
1.1 General
Read these instructions carefully before
using the product described within.
Should you have any questions about
these instructions or the use of this
product, please contact the customer
service department before using the
product:
Phone: +49 (7623) 7405 - 0
The product may only be placed in
service when its proper use can be
assured.
According to U.S. Standards,
PACE 203H is a Class III device
(21 CFR 862-892 [807.87(c)]).
According to European Standards,
PACE 203H is a Class IIb medical
product (Council Directive 93/42/EEC of
14 June 1993 (‘Medical Device
Directive’), Annex IX).
1.2 Checking the Delivery
Unpack the product and carefully check
to see if any damage has occurred
during shipment. Check to see if every-
thing was delivered as listed on the
shipping list. This includes:
•PACE 203H Dual-Chamber (DDD)
Temporary Cardiac Pacemaker /
External Pulse Generator
•9 V Alkaline Battery
•User’s Manual and Quick Reference
Guide.
Please inform OSYPKA AG immediately
if something is missing or damaged.
Claims that are filed afterwards will not
be considered.
1.3 Optional Accessories
(Not available in USA and Japan.)
The optionally available medical grade
AC power supply (100 ... 240 V / 50 ...
60 Hz) allows the operation of
PACE 203H while conserving battery
power.
The optionally available BPI 202™ intra-
aortic balloon pump interface provides
reliable ECG synchronization for intra-
aortic balloon pumps (IABP) which
otherwise rely on a surface ECG
obtained from three ECG electrodes.
1.4 Writing Conventions of
this Manual
In this instruction manual, certain con-
ventions are used. Keys and displays
are represented in the text as follows:
•Fixed labeled keys and dials are
marked with bold style:
ON, OFF, Pause,
Unlock/Lock
•Texts in the upper display are
marked with bold style:
AUTO, A-TRIG.
•Text in the lower display and soft-
labeled keys are marked in italics:
Main Menu, START