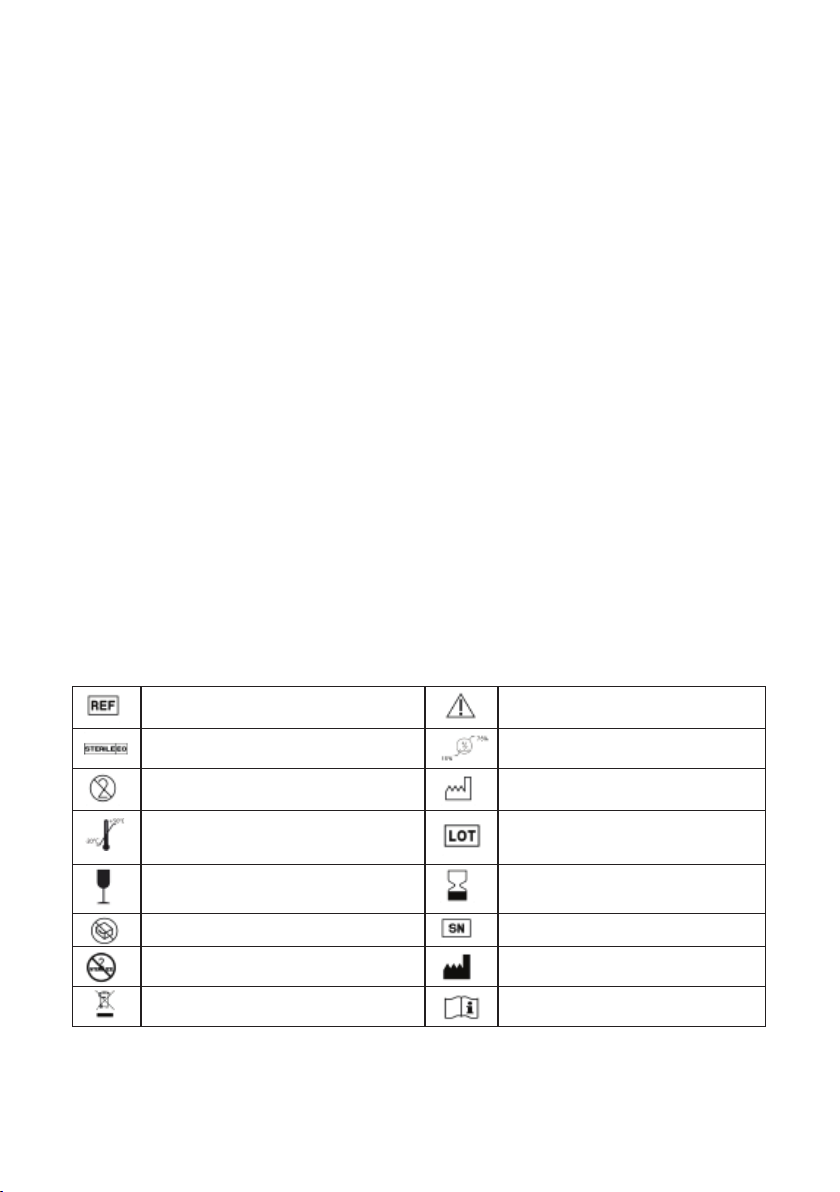
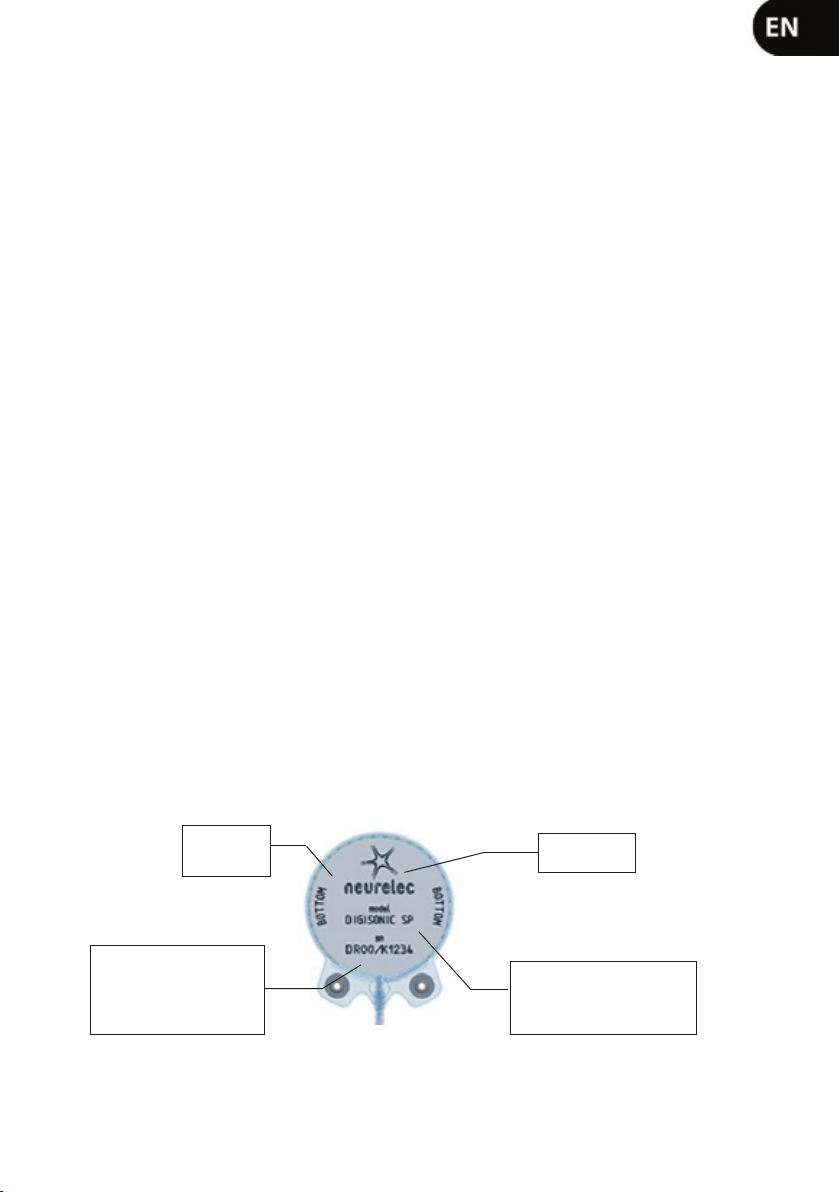
6. MEDICAL CONTRAINDICATIONS
The Digisonic®SP cochlear implant is not indicated in patients with perceptive hearing loss accompanied by
signicant lesions of the cochlea (major cochlear malformation, fracture of the petrous part of the temporal bone,
signicant ossication in the cochlea), auditory nerve (axonal neuropathy, tumor near or on the auditory nerve
such as a neurinoma, complete destruction of both auditory nerves), a severe anomaly of the auditory pathways,
acute or chronic middle ear conditions (including tympanic membrane perforation), is psychologically unstable
or has a contact allergy to implant materials (silicone, platinum iridium, titanium). Other types of implants may
be recommended.
7. ADVERSE SIDE EFFECTS
For patients who meet the indications, implantation has typical risks associated with surgery (eects from
general anesthesia, infections, etc.) which is independent of the product itself. However, there is also a risk
that the patient’s body may reject the implant or a part of the implant; this risk has been reduced by using
biocompatible materials in the design.
Complications associated with the cochlear implant operating technique (temporary or permanent facial
paralysis, risk of meningeal irritation syndrome, changes in taste, dizziness, tinnitus, etc.) are rare, but should
be considered carefully. It is important to inform every cochlear implant candidate about these potential risks.
Specic information should be given to the patient regarding the symptoms and the initial signs of meningeal
irritation syndrome. According to current recommendations, pneumococcal vaccination is also strongly
recommended.
Once the implant is in place, there remain certain risks that may result in explantation. Explantation requires
another surgical intervention under general anesthesia. Explantation may occur in the following cases:
- Medical complication
- Implant malfunction
- Displacement of the device as a result of trauma
- Extrusion of the implant
These potential problems were evaluated during product design and the materials and design of the implant
have been chosen to minimize these risks.
Finally, the long-term eects from trauma associated with the insertion of electrodes and chronic electrical
stimulation are unknown at the present time. These eects may include cochlear ossication or degeneration
of the nerve bers, and may require replacement of the implant or lead to a reduced response to stimulation.
8. PRECAUTIONS
Information to give the Patient
- Inform the patient of the benets of a cochlear implant, and also the adverse side eects which could occur
(see §7).
- The supplied identication card must be fully completed and given to the wearer.
- Inform the patient that they must present the identication card prior to any medical examination or treatment.
- Advise the patient to carefully read the user instructions supplied with his/her external processor, in particular
the section relating to the warnings for use.
- Tell the patient to contact the implantation center In case of failure or malfunction of the cochlear implant
system.
- Contact sports (rugby, boxing, etc.) are strongly discouraged, since strong impacts to the area of the implant
can damage it.
5