2
Contents
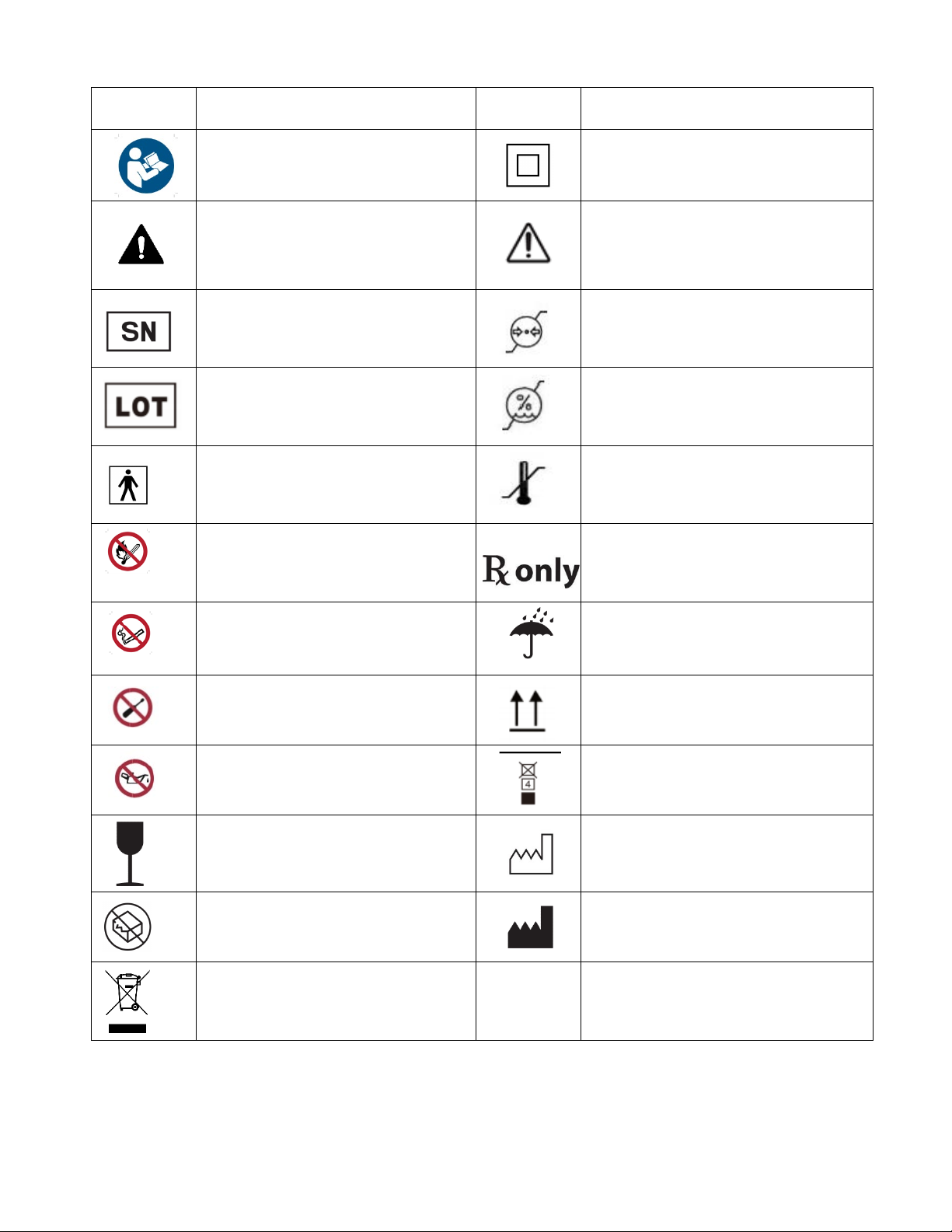
Glossary - Explanation of Packaging and Labelling Symbols.............................................................................3
Introduction...............................................................................................................................................................4
About OxyHome ....................................................................................................................................................4
General Information ..............................................................................................................................................4
How to Use this Manual........................................................................................................................................4
Intended Use..........................................................................................................................................................4
Contraindications and Precautions ....................................................................................................................4
Warnings Overview ..................................................................................................................................................5
Cautions Overview ...................................................................................................................................................6
Concentrator Features .............................................................................................................................................7
Front and Back of Concentrator..........................................................................................................................7
Side Panel ..............................................................................................................................................................7
Control Panel.........................................................................................................................................................8
User Controls.....................................................................................................................................................8
User Interfaces...................................................................................................................................................8
Indicator Lights..................................................................................................................................................8
Flow Meter..........................................................................................................................................................9
Oxygen Outlet Port............................................................................................................................................9
Accessories ...........................................................................................................................................................9
Operating Instructions .......................................................................................................................................... 10
General Instructions .......................................................................................................................................... 10
Cleaning, Care, and Maintenance ........................................................................................................................ 11
Routine Maintenance and Repairs ................................................................................................................... 11
Case Cleaning .................................................................................................................................................... 11
Cannula Replacement ....................................................................................................................................... 11
Filter Replacement ............................................................................................................................................. 11
Disposal of Equipment and Accessories ........................................................................................................ 11
Troubleshooting .................................................................................................................................................... 12
Product Specifications.......................................................................................................................................... 12
Environmental .................................................................................................................................................... 13
EMC Information .................................................................................................................................................... 13
TERMS AND CONDITIONS.................................................................................................................................... 17
Trademarks and Disclaimer .............................................................................................................................. 18
Trademark ....................................................................................................................................................... 18
Disclaimer ....................................................................................................................................................... 18
This Document................................................................................................................................................ 18
For Help ........................................................................................................................................................... 18