16
15
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures,
objects and people.
a. Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM
and FM radio broadcast and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the
measured field strength in the location in which the models KTR-230 are
used exceeds the applicable RF compliance level above, the models
KTR-230 should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such
as re-orienting or relocating the models KTR-230.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be
less than 3 V/m.
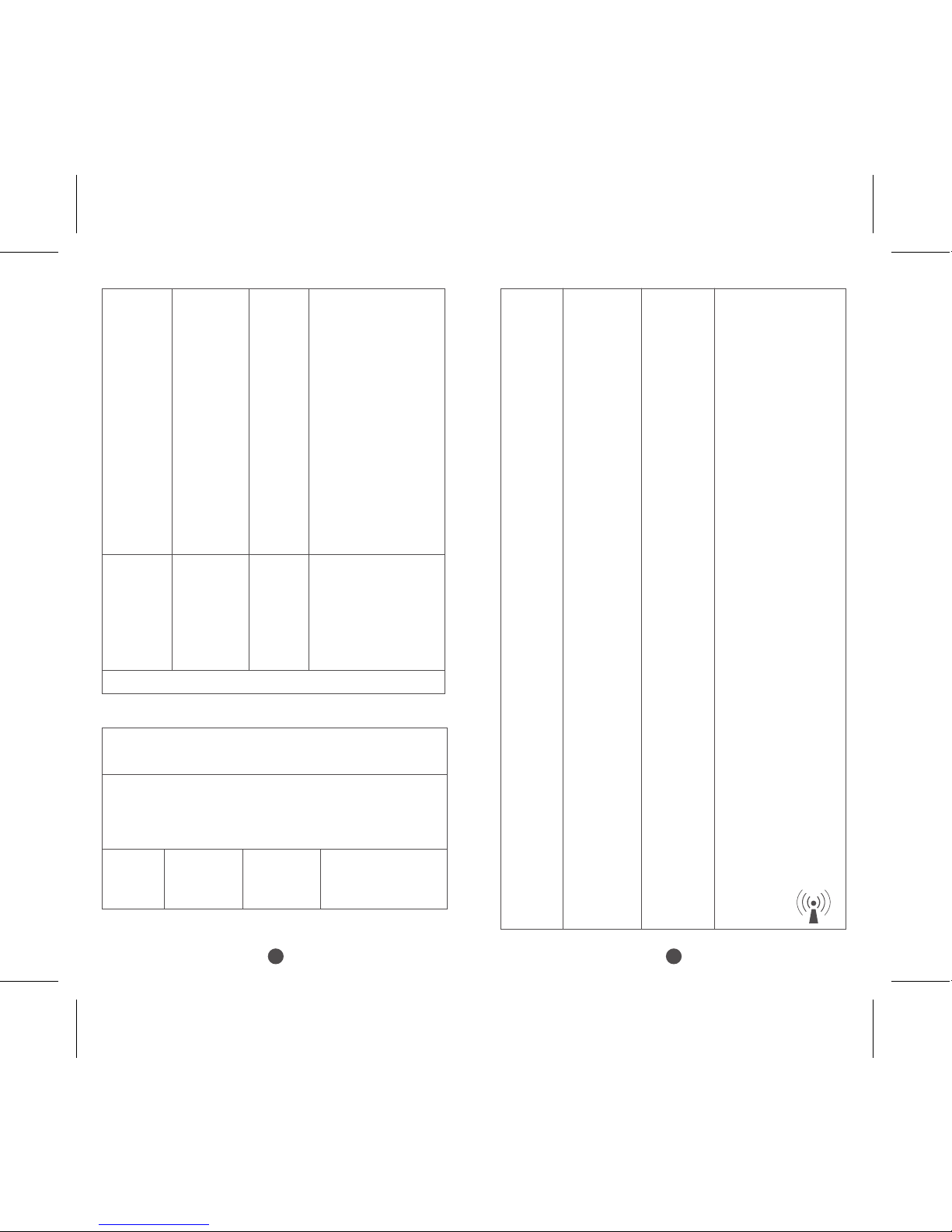
Recommended separation distances between portable
and mobile RF communications equipment and the models KTR-230
The models KTR-230 are intended for use in an electromagnetic
environment in which radiated RF disturbances are controlled. The
customer or the user of the MODEL KTR-230 can help prevent
electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment
(transmitters) and the models KTR-230 as recommended below,
according to the maximum output power of the communications
equipment.
Rated
maximum
output power
of transmitter
W
Separation distance according to frequency of transmitter
m
150kHz to 80MHz
d=1.2×P1/2
80MHz to 800MHz
d=1.2×P1/2
800MHz to 2,5GHz
d=2.3×P1/2
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power
not listed above, the recommended separation
distanced in metres (m) can be estimated using the
equation applicable to the frequency of the transmitter,
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation
distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations.
Electromagnetic propagation is affected by
absorption and reflection from structures, objects
and people.
Warranty
Please retain this warranty for reference in case after-sales repair or
maintenance is required.
The terms of the warranty are as follows:
Under normal operating conditions this product is covered by a
one-year warranty against manufacturing fault. In the unlikely
event of a manufacturing fault being encountered during this
period, the device will be repaired or replaced free of charge.
Cover is from the date of receipt.
Accidental damage or damage caused through misuse is not
covered by this warranty.
Shenzhen Kentro Medical Electronics Co.,Ltd
N0.3, Xihu industry zone, Xikeng Village, Henggang Town,
Longgang District, Shenzhen, Guangdong, China
Wellkang Ltd
Suite B, 29 Harley Street LONDON W1G 9QR, England, United
Kingdom Tel: +44 (20)30869438, 32876300 Fax: +44(20)76811874
0123